Sexual dimorphism in the Kudremukh Bush Frog (Anura: Rhacophoridae: Raorchestes tuberohumerus) of the Western Ghats, India, with a note on its distribution and conservation status
Anand D. Padhye 1, Anushree Jadhav 2, Shauri Sulakhe 3 & Neelesh Dahanukar 4
1,2 Department of Zoology, Abasaheb Garware College, Karve Road, Pune, Maharashtra 411004, India
2 Present address: Department of Zoology, Savitribai Phule Pune University, Ganeshkhind, Pune, Maharashtra 411007, India
3 Insearch Outdoors, C-26/9, Ketan Heights, Rahul Nagar Lane, Near Karve Statue, Kothrud, Pune. Maharashtra 411038, India
4 Indian Institute of Science Education and Research (IISER), G1 Block, Dr. Homi Bhabha Road, Pashan, Pune, Maharashtra 411008, India
4 Systematics, Ecology and Conservation Laboratory, Zoo Outreach Organization (ZOO), 96 Kumudham Nagar, Vilankurichi Road, Coimbatore, Tamil Nadu 641035, India
1anand.padhye@mesagc.org (corresponding author), 2anushreejadhav@gmail.com, 3shaurisulakhe@gmail.com, 4n.dahanukar@iiserpune.ac.in
doi: http://dx.doi.org/10.11609/JoTT.o4192.7211-22 | ZooBank: urn:lsid:zoobank.org:pub:3C420A17-847A-4947-B82F-982698C4F51C
Editor: Anonymity requested. Date of publication: 26 May 2015 (online & print)
Manuscript details: Ms # o4192 | Received 28 November 2014 | Final received 20 May 2015 | Finally accepted 21 May 2015
Citation: Padhye, A.D., A. Jadhav, S. Sulakhe & N. Dahanukar (2015). Sexual dimorphism in the Kudremukh Bush Frog (Anura: Rhacophoridae: Raorchestes tuberohumerus) of the Western Ghats, India, with a note on its distribution and conservation status. Journal of Threatened Taxa 7(6): 7211–7222; http://dx.doi.org/10.11609/JoTT.o4192.7211-22
Copyright: © Padhye et al. 2015. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and distribution by providing adequate credit to the authors and the source of publication.
Funding: This work was partially supported by DST-INSPIRE Research Grant [IFA12-LSBM-21] to Neelesh Dahanukar.
Competing Interest: The authors declare no competing interests. Funding sources had no role in study design, data collection, results interpretation and manuscript writing.
Author Contribution: ADP and SS collected specimens; SS performed photography; AJ performed morphometry; ADP performed osteology; ND performed genetic and statistical analysis; ADP and ND wrote the manuscript.
Author Details: Anand D. Padhye is an Associate Professor in Zoology, Abasaheb Garware College, Pune. He works on systematics, ecology, diversity, distribution and evolution of amphibians. Anushree Jadhav has completed her Masters in Biodiversity at Abasaheb Garware College, Department of Biodiversity. She is currently pursuing her M.Phil from Department of Zoology, Savitribai Phule Pune University. Shauri Sulakhe is a Founder-Director of Insearch Outdoors, an environment tourism organization in Pune. He is also a freelance field worker interested in wildlife photography and natural history. Neelesh Dahanukar works in ecology and evolution with an emphasis on mathematical and statistical analysis. He is also interested in taxonomy, distribution patterns and molecular phylogeny.
Acknowledgements: We thank Nikhil Modak for his help in field work. We are grateful to Dr. Asad Rahmani, Director; Dr. Deepak Apte, COO; Rahul Khot, incharge Natural History Collection department; Reshma Pitale, researcher and Vithoba Hegde, senior field assistant, for their help during study of the museum specimens from Bombay Natural History Society, Mumbai. We thank Dr. Sanjay Molur and Keerthi Krutha for their help in registration of specimens in the Wildlife Information Liaison Development Museum, Coimbatore. We are grateful to the Principal, Abasahab Garware College, Head Department of Zoology, Abasaheb Garware College and Indian Institute of Science Education and Research, Pune for providing infrastructure facilities. Anushree Jadhav is thankful to Dr. Narahari P. Gramapurohit for encouragement and support.
Abstract: Raorchestes tuberohumerus (Kuramoto & Joshy, 2003) was described based on three male specimens and was diagnosed mainly based on the presence of tubercle on the humerus. Here we describe the genetically confirmed female of the species and show that tubercle on the humeral bone is a sexually dimorphic character present only in males. Further, based on current collection and literature review we studied the distribution of the species using niche based modelling. Using the distributional range and our observations on the threats to the habitat we propose that Raorchestes tuberohumerus, currently assessed as Data Deficient, can fall under the ‘Vulnerable’ category of IUCN Red List of Threatened Species.
Keywords: Amphibians, IUCN Red List, molecular identification, osteology, threatened.
INTRODUCTION
Kuramoto & Joshy (2003) described the shrub frog Philautus tuberohumerus (now Raorchestes tuberohumerus vide Biju et al. 2010), based on three male specimens collected from Kudremukh, Western Ghats of Karnataka, India. Kuramoto & Joshy (2003) primarily diagnosed the species based on what they considered as the unique feature - the presence of a tubercle on the humeral bone, the etymological feature. While describing the species Raorchestes ghatei, Padhye et al. (2013) suggested that tubercle on the humeral bone of R. ghatei is a sexually dimorphic character present only in males and not females. However, such information was not available for R. tuberohumerus, because neither the first description of the species by Kuramoto & Joshy (2003) nor the subsequent revision of the group by Biju & Bossuyt (2009) had any female specimens in their study.
In the present study we provide the morphometry of genetically identified female specimens of R. tuberohumerus for the first time. Further, based on osteological study of both male and female specimens we show that the tubercle on the humerus is a sexually dimorphic character present only in the males. In addition, based on the niche-based modelling we predict the probable distribution of the species. Owing to the fact that the habitat of the species is under threat we suggest that the current assessment of ‘Data Deficient’ (Das 2004) be changed to ‘Vulnerable’ category on the IUCN Red List of Threatened Species (see assessment box at the end of the article).
MATERIALS AND METHODS
Study area and specimen vouchers
Specimens (four males and two females) were collected from Nidigere, in Karnataka State. Collected specimens are deposited in the museum collection of the Wildlife Information Liaison Development (WILD), Coimbatore and Abasaheb Garware College, Zoology Research Laboratory (AGC-ZRL), Pune, India. Additional specimens were studied from the museum collection of Bombay Natural History Society (BNHS), Mumbai, India.
Material examined
Raorchestes tuberohumerus (n=8): WILD-AMP-14-499 & 501 (males), WILD-AMP-14-500 & 502 (females), and AGC-ZRL-AMPHIBIA-201 & 202 (males), 29.vii.2014, (12.8480N & 74.8230E, 925m), on Madikeri-Sakleshpur Road, Nidigere Village, Karnataka, coll. Anand Padhye; Holotype, BNHS 4193, male, 15.vi.2000, from Kudremukh, Chikkamagalur, Karnataka, coll. by S.H. Joshy; Paratype, BNHS 4194, male, 15.vi.2000, from Kudremukh, coll. M. Kuramoto.
Morphometry and analysis
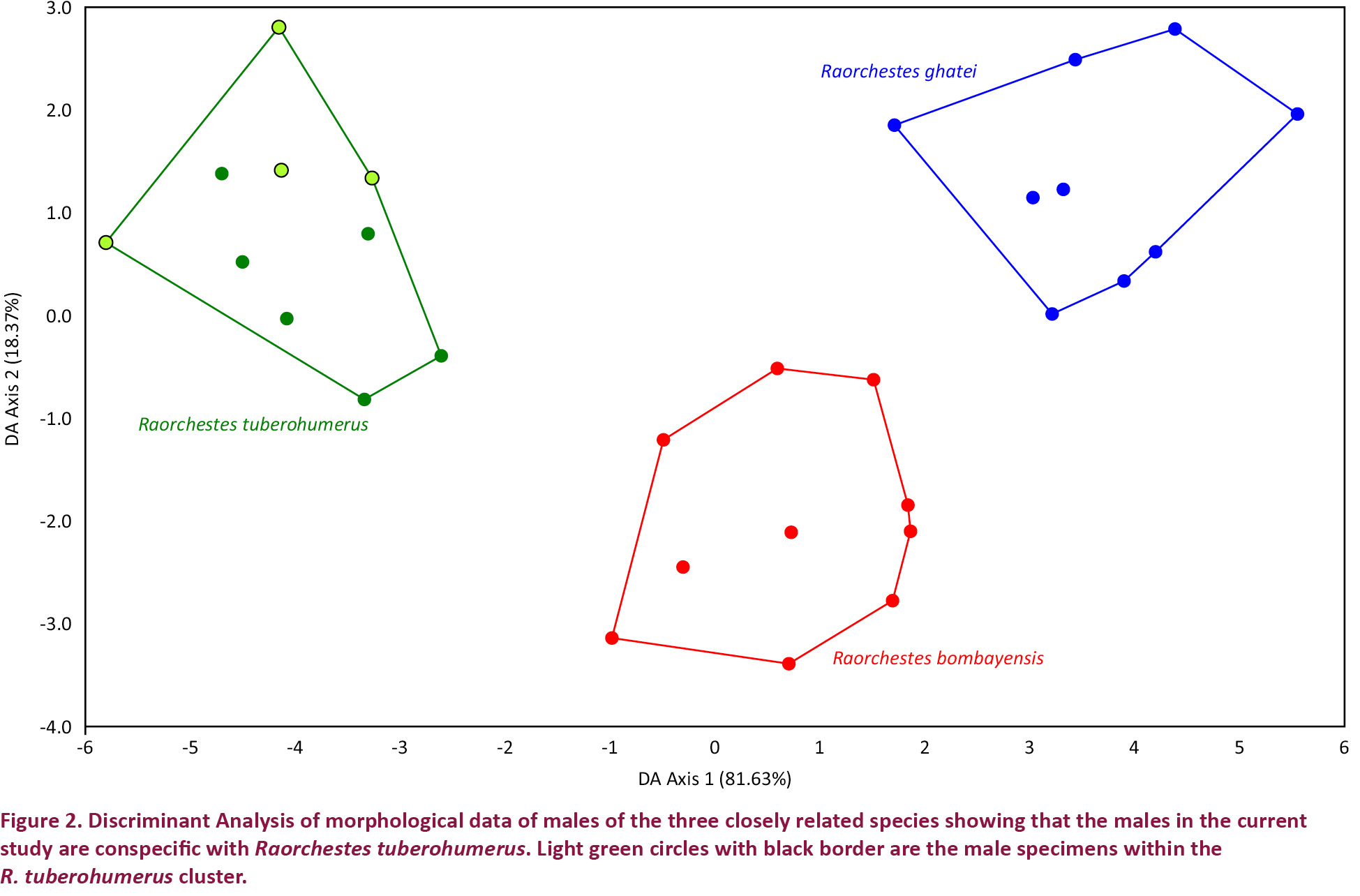
Measurements were taken to the nearest 0.1mm using a digital calliper and using a binocular microscope. The following measurement were taken (after Biju & Bossuyt 2009): snout-vent length (SVL); head length (HL); head width (HW); rear of the mandible to the nostril (MN); rear of the mandible to the anterior orbital border of the eye (MFE); rear of the mandible to the posterior orbital border of the eye (MBE); snout length (SL); nostril to tip of the snout (SN); front of the eye to nostril (EN); eye length (EL); inter upper eyelid width (IUE); maximum upper eyelid width (UEW); internal front of eyes (IFE); internal back of eyes (IBE); forelimb length (FLL); hand length (HAL); third finger length (TFL); disc width on finger III (FDIII); width of finger III (FWIII); shank length (ShL); thigh length (TL); foot length (FOL); distance from the heel to the tip of the fourth toe (TFOL). Tympanum diameter was measured both vertically (TYDV) and horizontally (TYDH). Data of the four males from current study were included in the data set used by Padhye et al. (2013) and discriminant analysis was performed in PAST (Hammer et al. 2001; free software) to confirm the morphometric identity of the species.
Osteology
Two specimens WILD-AMP-13-499 (male) and WILD-AMP-13-500 (female) were used for osteological study. Osteological clearing and staining procedure follow Potthoff (1984).
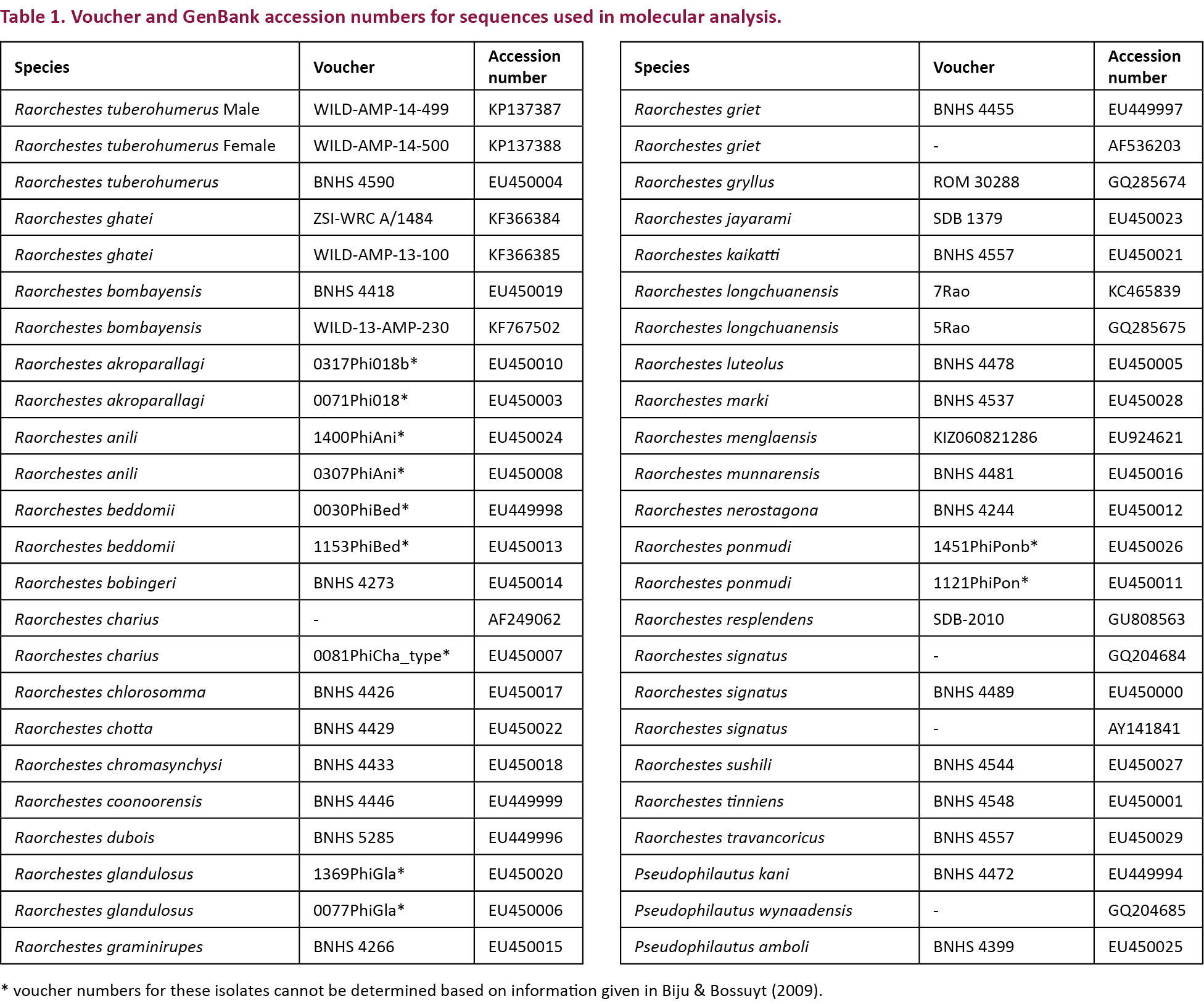
Genetic analysis
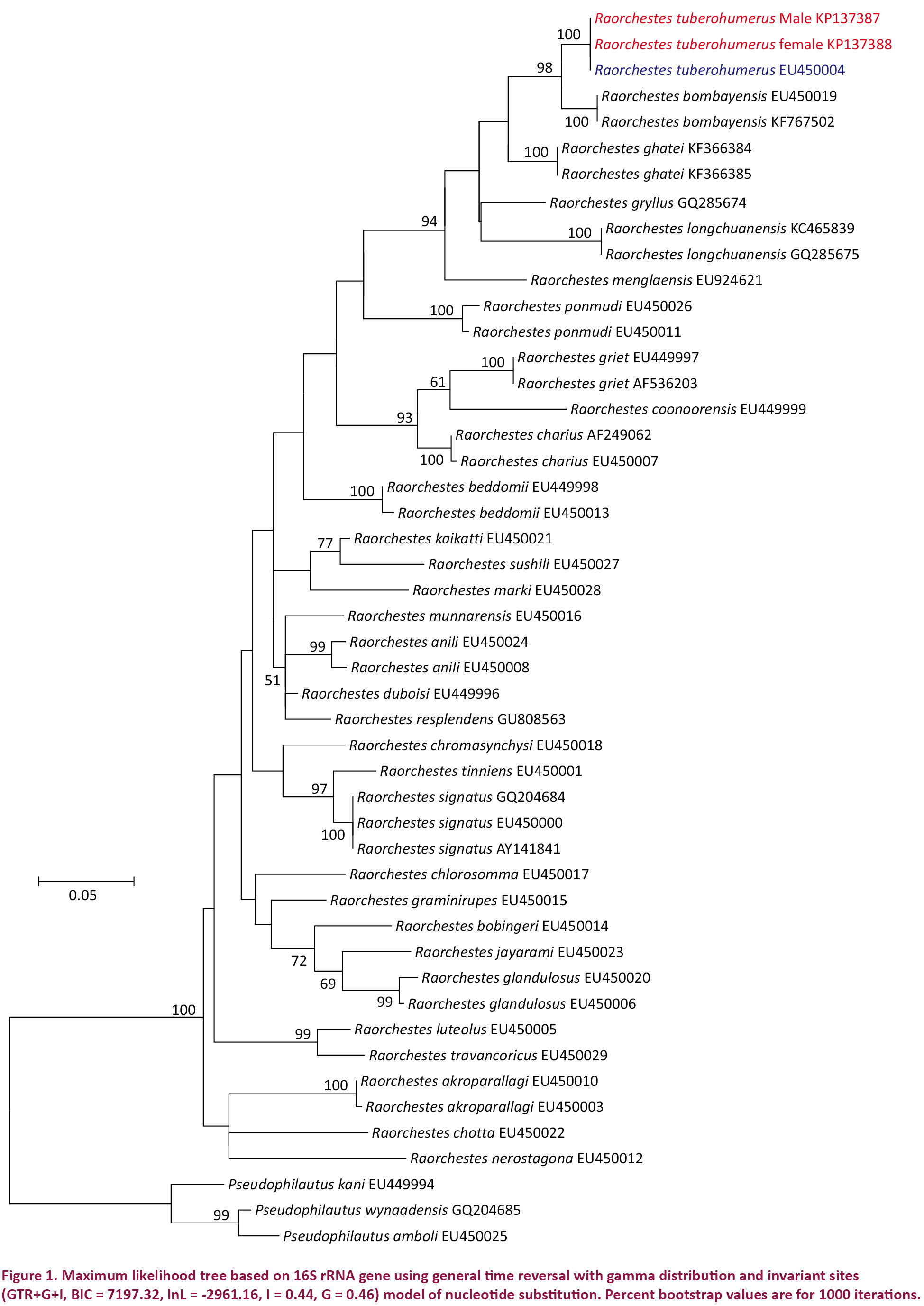
Muscle tissue was harvested from fresh specimens of a male (WILD-AMP-14-499) and female (WILD-AMP-14-500) and preserved in absolute ethanol. DNA extraction, PCR amplification of 16S rRNA gene and sequencing protocols are as per Padhye et al. (2013). Sequences were analyzed by BLAST tool (Altschul et al. 1990). These sequences have been deposited in GenBank (accession numbers KP137387 & KP137388). GenBank accession numbers for specimens used for analysis are provided in Table 1. Gene sequences were aligned using MUSCLE (Edgar 2004). Molecular phylogenetic analysis was performed using the freeware MEGA 6 (Tamura et al. 2013). The best fit model for nucleotide substitution was selected from 24 models using MEGA 6 (Tamura et al. 2013) based on the minimum Bayesian Information Criterion (BIC) value (Schwarz 1978; Nei & Kumar 2000). Best fit nucleotide substitution model was used to test the phylogenetic hypothesis using maximum likelyhood method implimented in MEGA 6 (Tamura et al. 2013). Genetic analysis was not carried out to thoroughly resolve the deep phylogeny of the genus but to assign individuals to genetically homogenous clusters for the purpose of species identification. Reliability of the phylogenetic tree was estimated using bootstrap values from 1000 replicates.
Prediction of distribution and calculation of extent of occurrence
Based on the present collection and point localities provided in Kuramoto & Joshy (2003), Das (2004), Biju & Bossuyt (2009), Gururaja (2012), Gururaja & Ramachandra (2012), we performed predictive niche-based distribution modelling to understand the probable distribution of R. tuberohumerus within the Western Ghats in the area between 8–22 0N and 70–80 0E. Predictive modelling was performed in DIVA-GIS (http://www.diva-gis.org/) using ~30 arc seconds data for altitude, precipitation and 19 bioclimatic parameters (Hijmans et al. 2005) available at the WorldClim website (http://www.worldclim.org/). Extent of occurrence (IUCN 2012) was estimated by overlaying the prediction map with hexagonal grid (Sahr et al. 2003) available online at the GLOBE website (http://globe.umbc.edu/documentation/foundational-data/globe-land-units-glus/). Each hexagonal cell has an area of approximate 100km2. All the grids which had some prediction were counted for determining the possible distribution of the species.
RESULTS AND DISCUSSION
Conformation of species identity
Genetic analysis based on 16S rRNA gene showed that the female specimen, without the tubercle on the humeral bone, and male specimen, with tubercle on the humeral bone, were genetically 100% similar to each other and showed no genetic divergence from the known sequence of R. tuberohumerus (GenBank accession number EU450004) (Fig. 1).
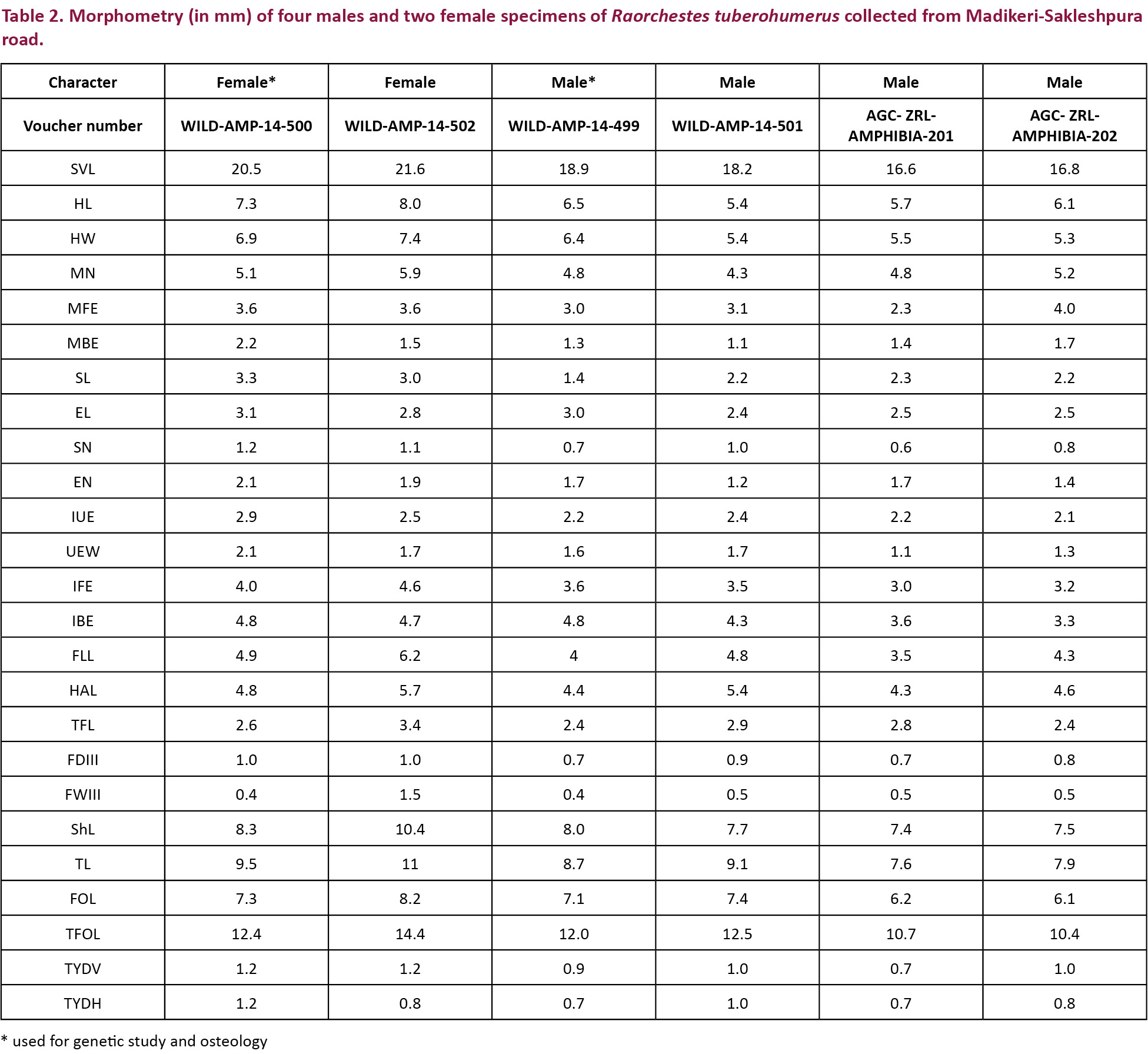
Male specimens collected from Madikeri-Sakleshpur road (Image 1) were morphologically similar to the holotype (BNHS 4193) and paratype (BNHS 4194) (both males) of Raorchestes tuberohumerus. Morphometric data (Table 2) of the males collected in the present study showed that the specimens clustered with the R. tuberohumerus (Fig. 2). We considered only males for morphometric comparison as earlier taxonomic studies (Kuramoto & Joshy 2003; Biju & Bossuyt 2009; Padhye et al. 2013) have considered only males while describing species in the genus Raorchestes. Further the morphometric data of females of R. bombayensis are not available and of R. tuberohumerus we have data of only two specimens in the current study. For R. tuberohumerus, males and females were morphologically similar apart from the larger size and absence of tubercle on the humeral bone in females.
According to Kuramoto & Joshy (2003), head is broader than long while according to Biju & Bossuyt (2009), head is as wide as long. However we have observed that head is slightly longer than wide or as wide as long in both males and females (Table 2). The difference in description of these characters, as compared to that of Kuramoto & Joshy (2003), is probably due to population variations or difference in methodology of morphometry.

Sexual dimorphism
Males posses a single, sub-gular vocal sac (Image 1b). We could not observe nuptial pads in males. Kuramoto & Joshy (2003) do not mention anything regarding nuptial pads in original species description; however, Biju & Bossuyt (2009) have mentioned the presence of slightly spinular nuptial pad in this species.
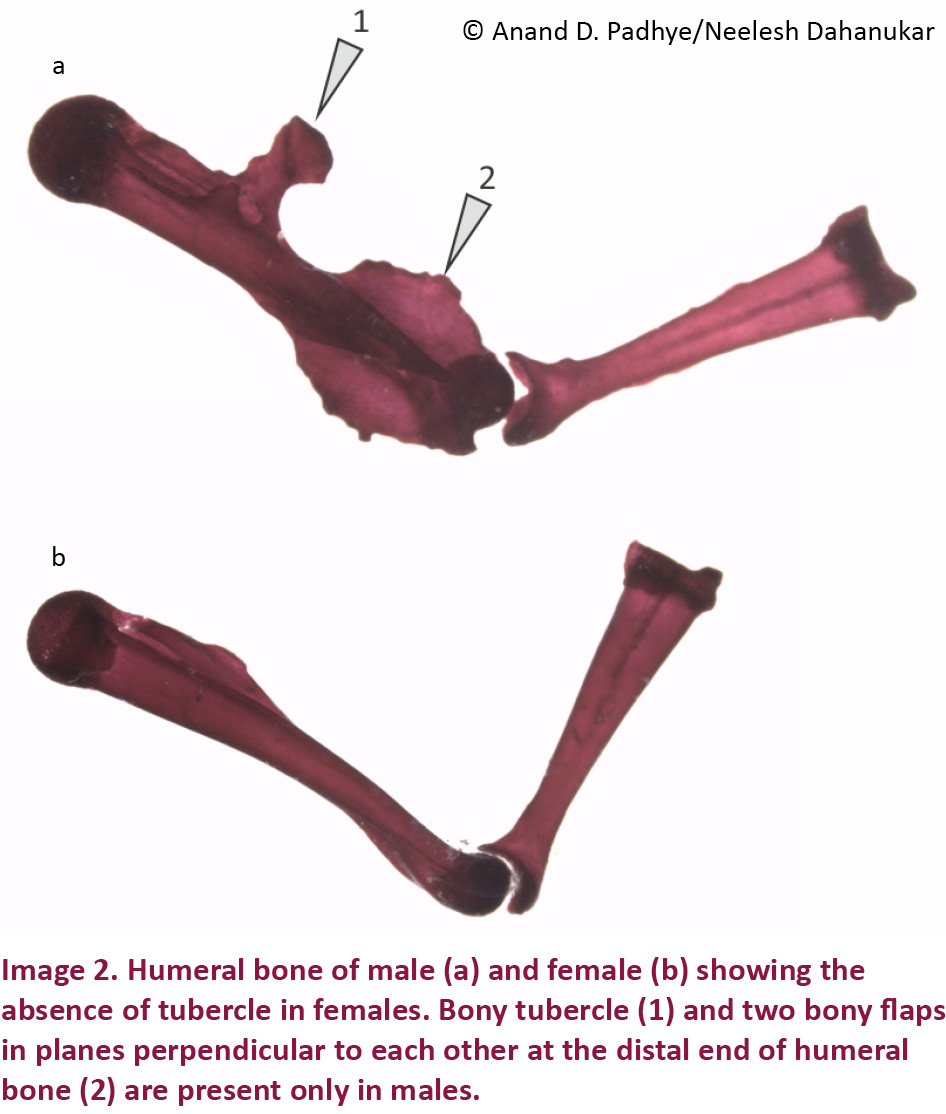
The most striking sexual dimorphism in this species is the presence of bony tubercle and two bony flaps in planes perpendicular to each other at the distal end of humeral bone in males and absent in females (Image 2). While describing R. tuberohumerus, Kuramoto & Joshy (2003) were not sure whether the presence of tubercle on humerus is male specific or not. In the revision of the taxa, Biju & Bossuyt (2009) also did not provide any information on the females of the species. Padhye et al. (2013), for the first time showed that similar to R. tuberohumerus, R. ghatei also possesses tubercle on the humerus; however, they noted the sexually dimorphic character present only in males. Padhye et al. (2013) predicted that even in R. tuberohumerus, the females may be devoid of the tubercle on the humeral bone. Current study confirms the humeral tubercle to be a sexually dimorphic character present only in males even in R. tuberohumerus.
A number of sexually dimorphic features in males are known in amphibians (Emerson & Voris 1992; Emerson 1996). However, modification of humeral bone as a sexually dimorphic character in bush frogs of the genus Raorchestes may have important ecological and evolutionary significance. Although, the exact role of the modified humeral bone is not clear, presence of tubercle as well as bony flaps in planes perpendicular to each other at the distal end of humeral bone may provide additional anchorage to the forelimb muscles which in turn could help in firm grip. The firmness of this grip was also noted by Kuramoto & Joshy (2003) who mention ‘…. the arms were folded under the chest when the frog was anesthetized, so tightly folded that it was hard to extend them’. Padhye et al. (2013) suggested that the modified humerus might have dual function: (1) clasping the females during amplexus, and (2) clinging to the small shrubs in windy habitats.
Based on our observations on the sexual dimorphism in Raorchestes tuberohumerus it is obvious that tubercle on the humerus cannot be used as a diagnostic character as the female cannot be identified by it. Currently, we are not aware of whether the modification of humeral bone in males is always present, however, given that this is an osteological character, it may not be a seasonal trait. Ecology and evolution of such a sexually dimorphic character in two different species of bush frogs, R. tuberohumerus and R. ghatei, calls for further studies in these aspects.
Species distribution, habitat, threats and conservation implications
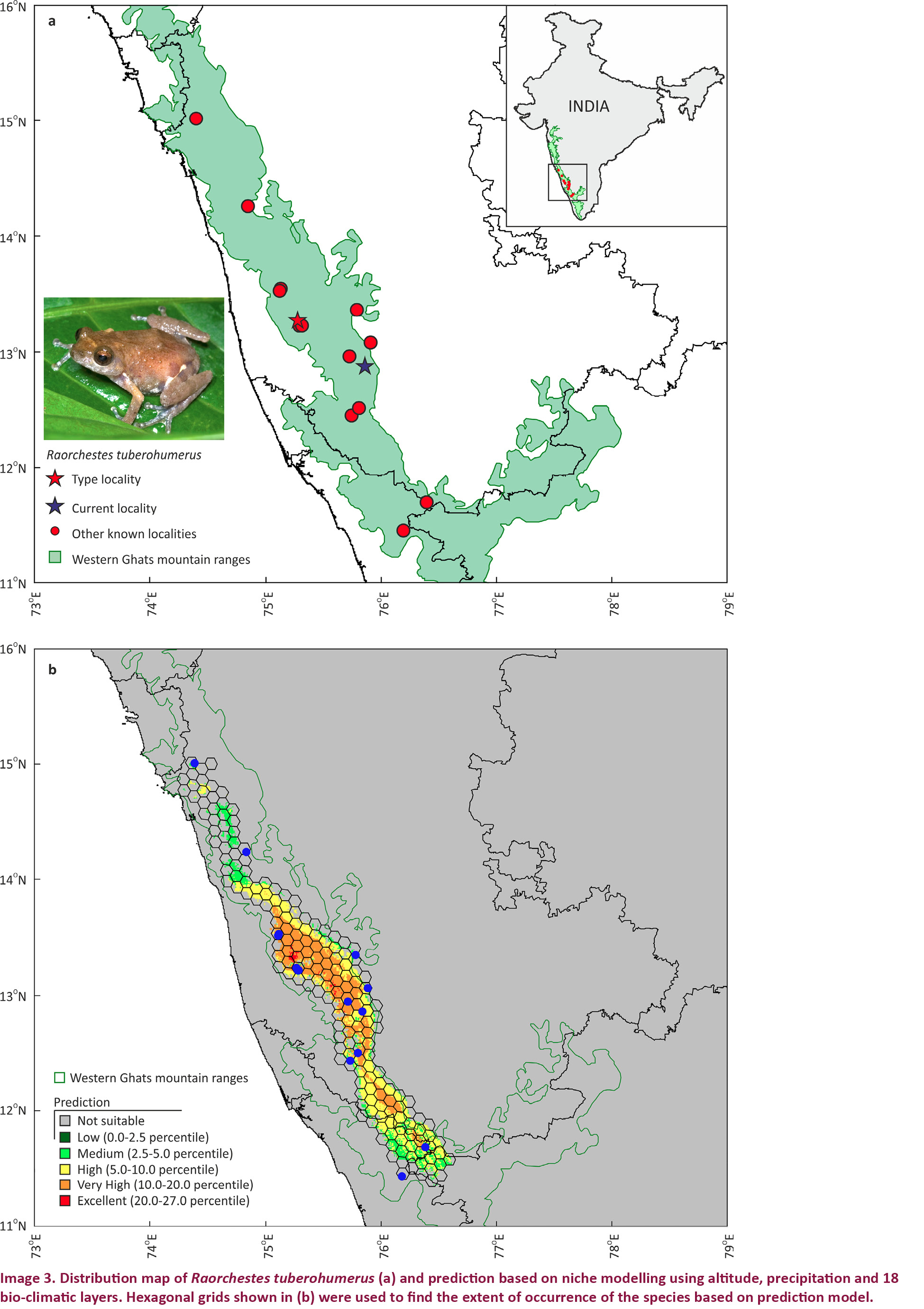
Raorchestes tuberohumerus is currently known to occur from 14 localities in the Western Ghats of Karnataka and northern Kerala (Image 3a) and include Anshi, Jog falls, Someshwar-Agumbe, Agumbe, Chikmagalur, Kudremukh, Malleshwaram, Kemphole, Sakleshwar, Madikeri-Sakleshpura Road, Kirundadu, Mercara, Muthunga and Wayanad (Kuramoto & Joshy 2003; Das 2004; Biju & Bossuyt 2009; Gururaja 2012; Gururaja & Ramachandra 2012). Gururaja (2012) has shown more points in the map for the distribution of this species but the point localities are not mentioned. Records of this species from Maharashtra (Padhye & Ghate 2012) are not considered as Padhye et al. (2013) have mentioned that these records should be assigned to R. ghatei. Niche-based prediction model suggests that the species is restricted to the Western Ghats mountain ranges (Image 3b). Hexagonal grids that coincide with at least some prediction were considered for finding extent of occurrence (EOO) (for explanation of EOO see IUCN 2012) and this accounted for an area of approximately 18,565km2. However, this was the maximum possible value as the prediction was not very high in all these hexagons. Considering only the very high prediction the EOO was estimated to approximate 10,966km2. The true EOO is likely to be between these two values. Currently, the species is known from 14 localities, which can fall under 10 severely fragmented locations (for explanation of locations see IUCN 2012) based on the threats to the habitat—(1) Anshi, (2) Jog Falls, (3) Someshwar-Agumbe & Agumbe, (4) Chikmagalur, (5) Kudremukh & Malleshwaram, (6) Kemphole, (7) Sakleshwar & Madikeri-Sakleshpura road, (8) Kirundadu & Mercara (Madikeri), (9) Muthunga, and (10) Wayanad.
We observed that Raorchestes tuberohumerus is found in the undergrowth on shrubs not more than 2m in height. Similar observations were made by Biju & Bossuyt (2009). The reproductive biology of this species is in Bossuyt et al. (2001). The species is found calling from a height of 0.5–2 m on the shrubs and lays 26 or 27 eggs on the leaves (Bossuyt et al. 2001). As the undergrowth forms the major habitat for the breeding of these species threat to the undergrowth can have severe effects on the species population. Clearing of undergrowth for land levelling and plantation could be the most important threat.
Information on species specific threats are not available for R. tuberohumerus, however, habitat destruction, change in land use pattern, heavy traffic, recreational activities and agricultural, organic and inorganic pollution are general threats to the habitat of the species in most parts of its distribution (Kumara et al. 2000; Seshadri et al. 2009; Gururaja & Ramachandra 2012; Ramchurjee 2013). Within some of the sanctuary areas (like Wayanad and Muthanga), habitats of this species could be threatened by the road widening activities associated with the national highway passing through. Even though the highways are closed at night, the highway traffic might contribute to the increased pollution level. Further, presence of several private lands at these sites are subject to cultivation and tourism, which may lead to the change in land use pattern and habitat modification / destruction. Plantations (in places like Anshi, Wayanad, Kirundadu and Madikeri) also pose threats through agricultural pollution through runoffs. In areas like Jog falls, Chikmagalur, Sakleshpur and Madikeri recreational activities due to heavy tourism, increasing urbanization and runoffs from spice and coffee plantations could affect the habitat of this species.
Recently, based on niche-based predictive modelling, Molur et al. (2015) suggested that this area of Western Ghats has a high propensity of Batrachochytrium dendrobatidis (Bd) infection. Because other species of Raorchestes of the Western Ghats are already known to be vulnerable to the infection by Bd (Dahanukar et al. 2013; Molur et al. 2015), it is possible that R. tuberohumerus is also likely to have Bd infection. Although it is not known whether Bd has impacted amphibian populations in the Western Ghats, Dahanukar et al. (2013) argued that “…….it is possible that the fungal infection may manifest in the near future through increased stressors such as organic and inorganic pollution, which might increase the virulence of the fungal strain and/or decrease the immunity of amphibian host”. If this is likely, then the presence of Bd could also be considered as a plausible threat to the species.
We do not have systematic sampling study to precisely pinpoint the threats to the populations of the species. Nevertheless, the fact that the species is restricted in distribution, has fragmented populations and there are several plausible threats to the habitat calls for raising conservation concern. Raorchestes tuberohumerus was assessed as Data Deficient (Das 2004) owing to lack of information. Based on the currently available data and above arguments we propose that the species is likely to be under the ‘Vulnerable’ category under the criteria B1ab(iii) for limited geographical range and threats to the habitat. A complete assessment is provided in Appendix A.
References
Biju, S.D., Y. Shouche, A. Dubois, S.K. Dutta & F. Bossuyt (2010). A ground-dwelling rhacophorid frog from the highest mountain peak of the Western Ghats of India. Current Science 98(8): 1119–1125.
Biju, S.D. & F. Bossuyt (2009). Systematics and phylogeny of Philautus Gistel, 1848 (Anura, Rhacophoridae) in the Western Ghats of India, with descriptions of 12 new species. Zoological Journal of the Linnean Society 155: 374–444; http://dx.doi.org/10.1111/j.1096-3642.2008.00466.x
Bossuyt, F., K. Roelants, L. Spithoven & M.H. Daro (2001). Philautus bombayensis (Bombay Oriental Shrub Frog) reproduction. Herpetological Review 32(1): 34–35.
Das, I. (2004). Raorchestes tuberohumerus. The IUCN Red List of Threatened Species. Version 2014.3. <www.iucnredlist.org>. Downloaded on 27 November 2014.
Edgar, R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792–1797; http://dx.doi.org/10.1093/nar/gkh340
Emerson, S.B. & H. Voris (1992). Competing explanations for sexual dimorphism in a voiceless Bornean frog. Functional Ecology 6: 654–660; http://dx.doi.org/10.2307/2389960
Emerson, S.B. (1996). Phylogenies and physiological processes—the evolution of sexual dimorphism in southeast Asian frogs. Systematic Biology 45: 278–289; http://dx.doi.org/10.1093/sysbio/45.3.278
Gururaja, K.V. (2012). Pictorial Guide to Frogs and Toads of the Western Ghats. Gubbi Labs Publication, 154pp+xviii.
Gururaja, K.V. & T.V. Ramachandra (2012). Anuran Diversity and Distribution in Dandeli Anshi Tiger Reserve. Sahyadri Conservation Series: 8. ENVIS Technical Report 37. Sahyadri: Environmental Information System (ENVIS), Centre for Ecological Sciences, Indian Institute of Science, Bangalore, India, 57pp.
Hammer, Ø., D.A.T. Harper & P.D. Ryan (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1): 1–9.
Hijmans, R.J., S.E. Cameron, J.L. Parra, P.G. Jones & A. Jarvis (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978; http://dx.doi.org/10.1002/joc.1276
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1. Second edition. Gland, Switzerland and Cambridge, IUCN, UK, iv+32pp.
Kumara, H.N., A.K. Sharma, A. Kumar & M. Singh (2000). Roadkills of wild fauna in Indira Gandhi Wildlife Sanctuary, Western Ghats, India: implications for management. Biological Conservation 3: 41–47.
Kuramoto, M. & S.H. Joshy (2003). Two new species of the genus Philautus (Anura: Rhacophoridae) from the Western Ghats, southwestern India. Current Herpetology 22: 51–60.
Molur, S., K. Krutha, M.S. Paingankar & N. Dahanukar (2015). Asian strain of Batrachochytrium dendrobatidis is widespread in the Western Ghats, India. Diseases of Aquatic Organisms 112: 251–255; http://dx.doi.org/10.3354/dao02804
Nei, M. & S. Kumar (2000). Molecular Evolution and Phylogenetics. Oxford University Press, New York, 333pp.
Padhye, A.D. & H.V. Ghate (2012). Amphibia. Fauna of Maharashtra, Zoological Survey of India State Fauna Series 20(1): 239–246.
Padhye, A.D., A. Sayyed, A. Jadhav & N. Dahanukar (2013). Raorchestes ghatei, a new species of shrub frog (Anura: Rhacophoridae) from the Western Ghats of Maharashtra, India. Journal of Threatened Taxa 5: 4913–4931; http://dx.doi.org/10.11609/JoTT.o3702.4913-31
Ramchurjee, N.A. (2013). Impacts of ecotourism in Rajiv Gandhi National Park (Nagarhole), Karnataka. Environment, development and sustainability 15(6): 1517–1525.
Sahr, K., D. White & A.J. Kimerling (2003). Geodesic discrete global grid systems. Cartography and Geographic Information Science 30 (2): 121–134.
Schwarz, G. (1978). Estimating the dimension of a model. Annals of Statistics 6: 461–464.
Seshadri, K.S., A. Yadav & K.V. Gururaja (2009). Road kills of amphibians in different land use areas from Sharavathi River basin, central Western Ghats, India. Journal of Threatened Taxa 1(11): 549–552; http://dx.doi.org/10.11609/JoTT.o2148.549-52
Tamura, K., G. Stecher, D. Peterson, A. Filipski & S. Kumar (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729; http://dx.doi.org/10.1093/molbev/mst197

