A failure of the Red Pierrot Talicada nyseus Guérin-Méneville, 1843 (Lepidoptera: Lycaenidae) butterfly to colonize Delhi area
Rajiv K. Singh Bais
A-13, Sector 33, Noida, Uttar Pradesh 201301, India
baisrajivsingh@gmail.com
doi: http://dx.doi.org/10.11609/JoTT.o3893.6920-6 | ZooBank: urn:lsid:zoobank.org:pub:0BBD1594-C691-4A0F-ABC0-56EEBEC1ADDC
Editor: B.A. Daniel, Zoo Outreach Organisation, Coimbatore, India. Date of publication: 26 February 2015 (online & print)
Manuscript details: Ms # o3893 | Received 30 December 2013 | Final received 22 December 2014 | Finally accepted 17 January 2015
Citation: Bais, R.K.S. (2015). A failure of the Red Pierrot Talicada nyseus Guérin-Méneville, 1843 (Lepidoptera: Lycaenidae) butterfly to colonize Delhi area. Journal of Threatened Taxa 7(2): 6920–6926; http://dx.doi.org/10.11609/JoTT.o3893.6920-6
Copyright: © Bais 2015. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and distribution by providing adequate credit to the authors and the source of publication.
Funding: None.
Competing Interest: The author declares no competing interests.
Acknowledgements: Author is thankful to Dr. Gurcharan Singh of Delhi University for providing information about status of Kalanchoe in Delhi area. Author also thanks the editor Dr B.A. Daniel and two anonymous referees for critically examining and offering useful comments on the original manuscript.

Abstract: The Red Pierrot Talicada nyseus Guérin-Méneville, 1843 is a butterfly of the semi-arid plains. Its historical distribution range includes Sri Lanka, southern India, northeastern India and Myanmar. It was recently reported from a few places in northern India far away from its known range boundaries in India. Dehradun, Kumaon Himalaya, Delhi and Kalatop are the places from where it has been reported in the recent past. Talicada nyseus nyseus appeared in the Delhi area in 2008, survived and bred for more than a year and then suddenly disappeared in the summer of 2009. The appearance of T.n. nyseus in Dehradun has been linked to the introduction of Kalanchoe ornamental plants in the newly developed residential areas. This paper examines the likely reasons for its disappearance from the Delhi area.
Keywords: Common Indian Yellow Wasp, Kalanchoe, Polistes hebraeus, Red Pierrot, Talicada nyseus.
The Red Pierrot (Talicada nyseus Guérin-Méneville, 1843) is a butterfly of semi-arid plains, human habitations, urban gardens, hill stations and forests where Kalanchoe occurs (Kunte 2000). The nominate subspecies Talicada nyseus nyseus is distributed in peninsular India south of Mumbai and Odisha. Two other subspecies are found in Assam and Burma (Myanmar) (Larsen 1987). Its historical distribution range includes Sri Lanka, southern India, northeastern India and Myanmar (see Table 1). It was recently reported from Dehradun where it is present only in the urbanized blocks with ornamental Kalanchoe plants in home gardens but absent from the native vegetation of the area (Singh 2005). It was also reported from Kumaon Himalaya (Haldwani, Chandadevi, Jones Estate) where it is now well established (Smetacek 2011). It was also reported from Delhi (Smetacek 2009) and Kalatop in Himachal Pradesh (Mahendroo 2013).
To this list I add my own two records of this butterfly from northern India from outside its known historical range: one butterfly was spotted on 29 May 2008 at Kataha Ghat (27.106173N & 81.940446E), Gonda (Uttar Pradesh) and one on 31 May 2008 at Women’s Polytechnic Campus, Lucknow (26.87650N & 81.000931E). I photographed the butterfly in Lucknow for the sake of a record and made an entry in my diary for the Gonda observation.
In Delhi, I first spotted this butterfly on 16 March 2008 at Lodi Colony and later on 26 March 2008 in Noida. Soon it became a regular sight in Noida and I started keeping a record of its sightings. On 07 June 2008, I collected caterpillars of T. n. nyseus and brought them home. The first butterfly emerged on 15 June 2008. I released it outside after taking photographs. On the very same day the monsoon reached Delhi, breaking a 108-year-old record (The Hindu, 16 June 2008) of early arrival. The scheduled normal date of arrival of the monsoon in Delhi is 29 June. On the very first day of the monsoon Safdarjang meteorological office recorded 30.3mm of rainfall between 08.30hr and 17.30hr. However T. n. nyseus disappeared from Delhi/Noida sometime in the middle of the summer of 2009 and has not been spotted since then.
Study area
Sectors 33 and 24 of Noida (28.60N & 77.35E), India. Noida is a satellite town of Delhi. Sector 33 is a residential area with small home gardens and potted plants in almost all the houses. Sector 24 is an Institutional Area with numerous offices and educational institutes. A busy main road separates the two sectors. There are numerous Kalanchoe plants in home gardens and also in the flower beds in the office.
Methods
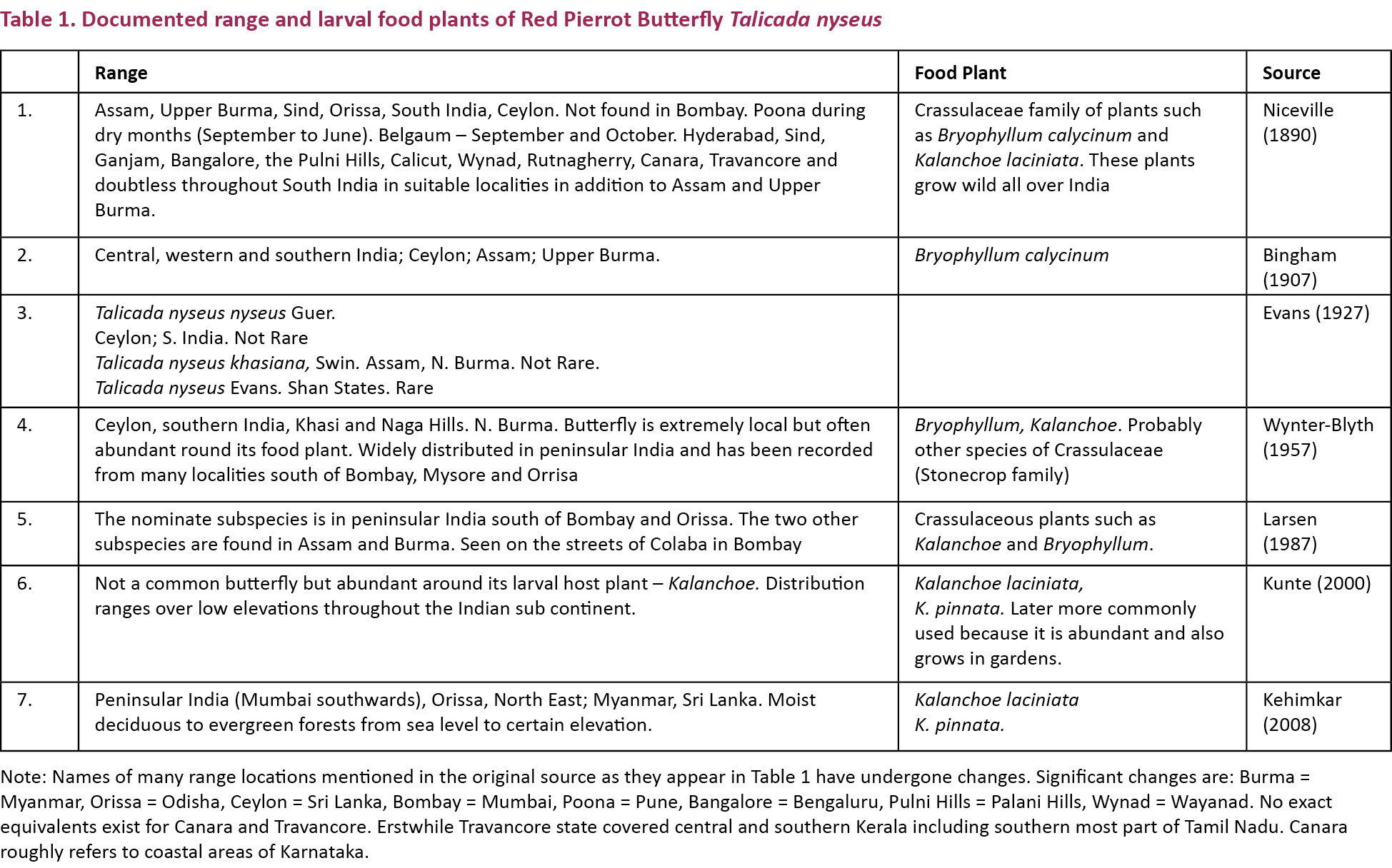
I kept daily records of butterfly sightings, including T. n. nyseus around my home in Sector 33 Noida for the period (2003–2012). Never in large numbers, this butterfly was seen only around the potted Kalanchoe plants in the home gardens in front of the houses. Fig. 1 shows the maximum number of the butterfly observed any month from January 2008 to December 2009.
After reading the initial reports of its survival in Dehradun, I believed it would easily establish itself in the Delhi area also but when it went missing for four consecutive years after its last sighting in May 2009, I thought it fit to carefully examine the reasons for its disappearance from the Delhi area. I followed the following basic model to get an insight into the ecological requirements of this butterfly:
(i) List all the known larval host plants of T. n. nyseus reported till date
(ii) Plot a global distribution of these host plants
(iii) Tabulate distribution of larval food plants in India
(iv) Check availability of these host plants in the Delhi area
(v) Plot current global distribution of T. n. nyseus .
(vi) Analyze the patterns emerging from the above superimposed maps.
(vii) Propose likely reasons for the disappearance of this butterfly from the Delhi area.
Larval host plants
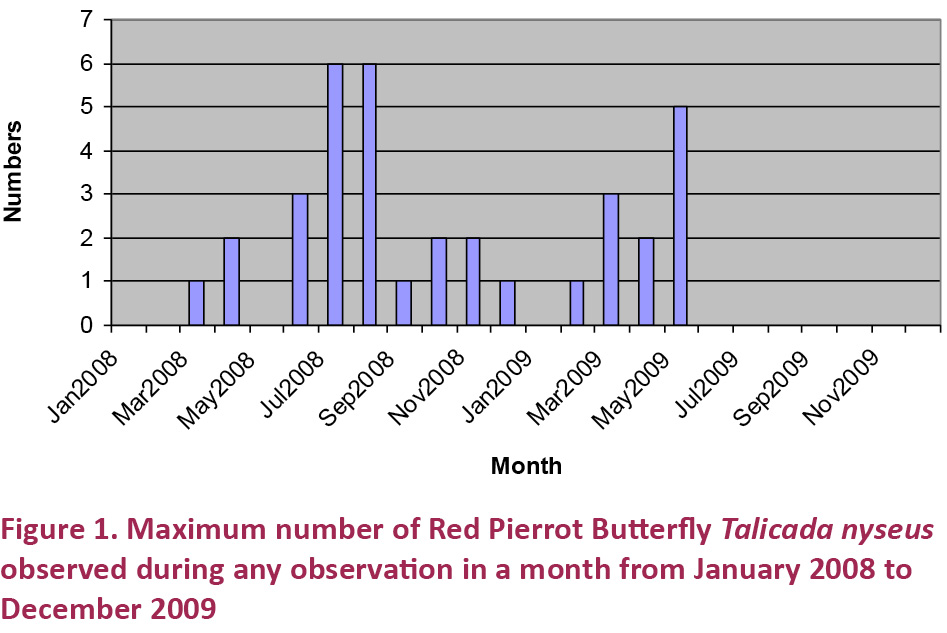
The presence of larval food plants is a fundamental requirement for the survival of any butterfly species in any particular geographical area. Though the mere presence of larval food plants of any specific butterfly species in an area does not guarantee the presence of that butterfly in the area, an absence of such food plants positively rules out their presence from the area. All the known larval food plants for T. n. nyseus are presented in Table 1. It becomes very clear that the T. n. nyseus larva feeds exclusively on Kalanchoe species.
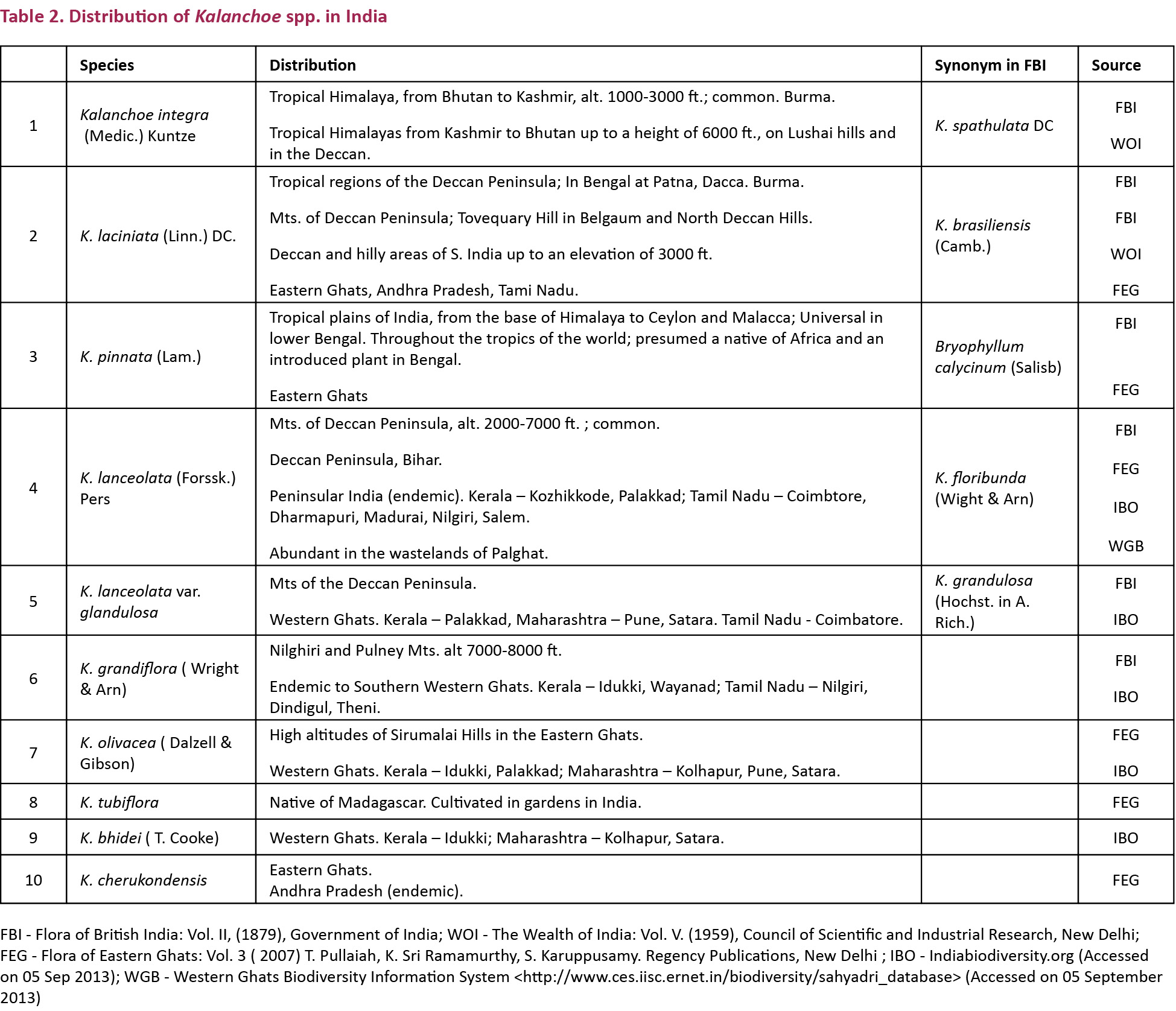
Kalanchoe (Crassulaceae) comprises about 125 species of succulent flowering plants. With one species in America, 56 in southern and eastern Africa, and 60 in Madagascar, the genus extends to south-eastern Asia and as far as China. Madagascar contains the largest number of species, suggesting that it is an ancient centre of speciation, and perhaps the centre of origin for the genus (Allorge-Boiteau 1996). As per The Wealth of India - Raw Materials Vol. V, about 11 species are recorded from India though it (The Wealth of India) includes only three: Kalanchoe integra, K. laciniata, and K. pinnata, and further says that many Kalanchoe species are grown in India for their ornamental foliage and flowers. They prefer dry, rocky and sandy localities and are useful in gardens as border and pot plants and in rockeries. Table 2 lists 10 Kalanchoe species found in India in the wild. Until about 180 million years ago (Mya) the Indian plate was still joined with the Madagascar and Madagascar in turn was connected to Africa, so the presence of about 10 Kalanchoe species in peninsular India is no surprise.
Likely origin of Talicada nyseus nyseus
Although it is tempting to assume that T. n. nyseus in peninsular India is a part of the remnant original fauna of the Madagascar and that the butterfly rode the Indian plate on its journey north, one study (Kunte 2011) of the biogeographic origins of the butterflies of the Western Ghats shows that the butterfly fauna of Western Ghats was established fairly recently in comparison to the tectonic history of the Indian peninsula. It further quotes (Larsen 1984) that dry habitat genera which includes Tarucus are most likely of Eremic (Saharan and central Arabian) origin which spread to India relatively recently, certainly long after the initial collision of the Indian plate with the Eurasian plate. The Indian plate collided with the Eurasian plate about 45 Mya.
In either case the presence of contiguous habitats throughout the path of its terrestrial dispersal is necessary because its very weak flight close to the ground suggests that it is unlikely to fly long distances across large oceans.
Discussion
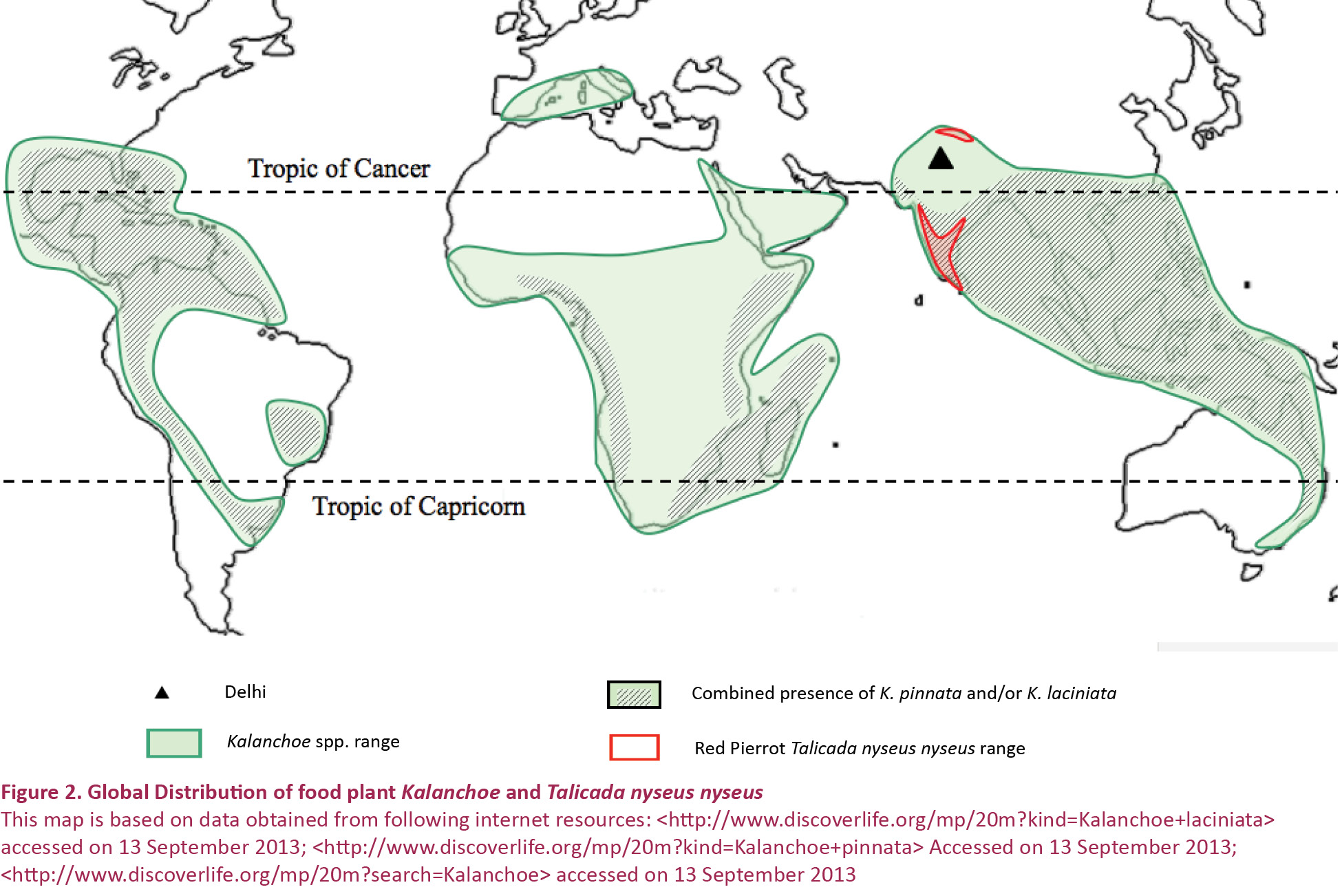
Larsen (1987) says that T. n. nyseus is locally abundant in the Nilgiri mountains of southern India from the foot of the ghats to the highest peaks; also present on the streets of Colaba in Bombay and its only ecological requirement seems to be the presence of Crassulaceous plants such as Kalanchoe and Bryophyllum. Its recent discovery in Dehradun (Singh 2005) was also linked to the introduction of Kalanchoe in home gardens in Dehradun which reinforced the observations made by Larsen. Fig. 2 shows the global distribution of Kalanchoe and also of T. n. nyseus. Keeping aside all other ecological factors limiting the spread and survival, the theoretical geographical range of T. n. nyseus can be as large as the entire tropical regions of the world wherever Kalanchoe is present. The present distribution of T. n. nyseus is much smaller. Even if we assume that most species of Kalanchoe can not be eaten by the T. n. nyseus larvae but only Kalanchoe pinnata and K. laciniata, as recorded from India, then also the butterfly should have expanded its range to the areas where Kalanchoe pinnata and K. laciniata occur, but it failed. Factors other than food plant availability restrict the range of T. n. nyseus to a much smaller geographical area which describes a very specific niche required for the survival of this butterfly. It implies that we can not simply bring in caterpillars of this butterfly to Delhi embedded in the fleshy Kalanchoe and expect them to survive unless other ecological factors are also equally favourable, including interactions with predators and parasites.
At least one Kalanchoe species, namely Kalanchoe integra (Medic.) Kuntze (K. spathulata DC) occurs naturally in the Himalaya from Kashmir to Bhutan which may explain the sustenance of recently introduced (or discovered) T. n. nyseus around human habitations in Dehradun, Kumaon and Kalatop. But the case with Delhi is different.
Status of Kalanchoe in Delhi area
Table 2 shows that there is no natural or naturalized Kalanchoe species in Delhi area, though ornamental plants of Kalanchoe species are present in public parks and home gardens. This was also confirmed by Dr. Gurcharan Singh (pers. comm. 2013) of Delhi University. According to Gurcharan Singh in Delhi only cultivated species of Kalanchoe are found which he had personally identified and photographed and these included: Kalanchoe beauverdii, K. beharensis, K. blossfeldiana, K. daigremontiana, K. pinnata, K. thyrsiflora and K. tomentosa. Gurcharan Singh wrote in the same communication that J.K. Maheshwari’s Flora of Delhi does not include Kalanchoe; not even the family Crassulaceae.
The quality of the habitat is the highest in the centres of species’ ranges and becomes more unsuitable as the edge of the range is approached and the highest population densities are observed in the range core, but the species become increasingly rare towards its range margin. Without frequent immigration from core populations to replenish individuals lost in peripheral populations, the latter may exhibit an increased incidence of local extinction (Mott 2010). It needs to be kept in view that T. n. nyseus is a weak flier and is generally seen fluttering around the food plant close to the ground. So, if it is purposely or accidentally introduced in the Delhi area then its survival will depend on the presence of a Kalanchoe-rich corridor up to the nearest wild population of Kalanchoe species in the forests of Himalaya or Deccan so that heavily preyed upon butterflies can be quickly replaced by fresh arrivals from the original source until the local population stabilizes. Without this it is unlikely that T. n. nyseus will ever survive in the Delhi area.
Talicada nyseus nyseus survived in the Delhi area for one full season, bred and produced a new generation shows that there are numerous favourable factors; biotic, abiotic or other, helping this butterfly to get a strong foothold in a new territory. However its sudden disappearance in May 2009 points to certain very unfavourable factors not allowing it to get fully established in the Delhi area.
Predation by Indian Yellow Wasp
Predation by the Indian Yellow Wasp (Polistes hebraeus Fabr.) appears to be one such factor. On 17 May 2009 a mating pair of T. n. nyseus was caught by a P. hebraeus in Sector 24 (Images 1–3). As the wasp devoured one of the butterflies of the coupled pair the other one still remained joined until the piercing mandibles of the predator reached the junction point and then, with some struggle, the second butterfly succeeded in de-coupling itself and flew away. The wasp continued chewing the abdomen of the killed butterfly after discarding the curled up wings. When only a small ball of flesh remained, then the wasp carried it away to some unknown destination; most likely to its nest as food for the young. This was my last observation of this butterfly before it completely disappeared from the area.

In India P. hebraeus often occurs in and around dwellings. This wasp constructs papery nests in verandahs, eaves, ceilings or rafters or any convenient spots (The Wealth of India, Vol. V). In Noida, nests of Yellow Wasp are seen located 3–5 m from the ground, on small trees. The leafy canopy of the tree provides necessary protection against the sun and rain. Inside the houses these nests are found inside the window mounted room water coolers, under flights of stairs or even inside the silencer of a parked motorcycle if it is not used for long. During spring time there are normally 3–4 smaller circular nests of P. hebraeus in our home measuring just 5cm across. Nest building begins around Holi festival in the month of March and the entire process of nesting and breeding slowly comes to an end after the monsoon hits Delhi. In 2010 a big rectangular nest was constructed inside our house measuring 23x12 cm with around 800 cells. It hung from the lintel of the corner window of the bathroom on the outside.
Polistes spp. are generalist predators. Adult wasps forage for two classes of food: arthropods and nectar (Gadagkar 1991). Caterpillars are the chief source of food for the young of these wasps. In one study (Clapperton 1999) conducted in New Zealand on recently established Asian Paper Wasp (Polistes chinensis) lepidopteran larvae were found to be the most common prey accounting for 90% of the arthropods identified in the collected pellets. Other arthropod groups represented were Diptera, Coleoptera, Formicidae, Cicadellidae and Pholicidae. For searching caterpillars, workers descend on the grass, herbs and shrubs and meticulously search each and every blade of grass by first landing on the blade and then slowly walking the entire length, first on the top and then on the underside, stopping now and then on sensing the presence of any caterpillar and then finally lifting the caterpillar and flying back to the nest. Bigger caterpillars are killed on the spot and only smaller flesh balls are transported. T. n. nyseus caterpillar is protected against such attacks from wasps and other predators as it bores a hole in the fleshy leaf of the Kalanchoe as soon as it emerges from the egg shell and enters inside of the leaf to spend the rest of its life in complete safety between the two epidermal layers of the leaf. However the adult butterfly has no such protection.
In Delhi, T. n. nyseus thrived only around potted Kalanchoe plants in the home gardens and could never get a foothold in the wild for want of larval food plants. However these very home gardens were also natural homes to the abundantly available ubiquitous P. hebraeus which easily captured these slow flying butterflies for feeding to their young during the breeding season. So, it appears that probably the T. n. nyseus population may stabilize in Delhi area away from man-made buildings and structures where predation pressure from P. hebraeus is much less or altogether absent. One location that immediately comes to mind is the rocky area of the Delhi Ridge around Indira Gandhi National Open University (IGNOU) in Maidan Garhi with very few tall trees and only shrubs and rocks strewn all over the place. If Kalanchoe is planted around these rocks in large areas then probably the butterfly will survive.
But how did it survive predation pressure in 2008? One study (Dejean 2011) shows a very strong decline in wasp populations due to particularly heavy rainfall during the main rainy season in neotropical social wasps and also an increase in their populations as a result of low rainfall during the main rainy season. Early and excessive rainfall of 2008 from May to September in Delhi seems to have suppressed nesting and foraging activities of the P. hebraeus whereas T. n. nyseus flourished after its initial arrival in March 2008. In 2008, the monsoon advanced over Delhi on 15 June, about two weeks earlier than the normal date (29 June). During June, the northern parts of the country received excess rainfall. Punjab, West Uttar Pradesh and Haryana, Chandigarh and Delhi taken together received more than three times their normal rainfall (Monsoon Report 2008). Moreover, even before the monsoon reached Delhi the month of May recorded 146.2mm of rain, more than the total rainfall received in June (Indian Meteorological Department, District Rainfall Data for Delhi). This kind of excess rainfall must have suppressed the wasp population. But just the opposite happened in 2009. Year 2009 was the third highest rainfall deficient monsoon season (June to September) in the last 109 years for India (1901–2009). For entire India it was 22% deficient but for Haryana, Chandigarh and Delhi sub-division it was 35% deficient (Monsoon Report 2009). With little rains in May and practically rainless, hot and bright June, nesting and foraging activities of P. hebraeus in the summer of 2009 must have increased considerably resulting in heavy predation pressure on the lepidopteran caterpillars and adult butterflies. This predation, coupled with the fact that there are no wild populations of T. n. nyseus around Delhi to replenish the lost population, appears to be one of the major factors for the disappearance of this butterfly from the Delhi area.
References
Allorge-Boiteau, L. (1996). Madagascar centre de speciation et d’origine du genre Kalanchoe (Crassulaceae). Biogeographie de Madagascar 137–145pp.
Bingham, C.T. (1907). The Fauna of British India: Butterflies - Vol II. Taylor and Francis, London, viii+480pp.
Clapperton, B.K. (1999). Abundance of Wasps and prey consumption of Paper Wasps (Hymenoptera, Vespidae: Polistinae) in Northland, New Zealand. New Zealand Journal of Ecology 23(1): 11–19.
Dejean, A., Ce´re´ghino, R., Carpenter, J.M., Corbara, B., He´ rault, B., Rossi V., Leponce, M., Orivel. J., & Bonal, D.(2011) Climate Change Impact on Neotropical Social Wasps. PLoS ONE 6(11): e27004; http://dx.doi.org/10.1371/journal.pone.0027004
Evans, W.H. (1927).The Identification of Indian Butterflies. Bombay Natural History Society, Bombay, xi+302pp.
Gadagkar, R. (1991). Chapter 5: Belonogaster, Mischocyttarus, Parapolybia, and Independent-founding Ropalidia, pp. 149–190. In: Ross, K.G. & R.W. Matthews (eds.). The Social Biology of Wasps, Cornell University Press, Ithaca, United States.
Kehimkar, I. (2008). The Book of Indian Butterflies. Oxford University Press, India, xvi+497pp.
Kunte, K. (2000). Butterflies of Peninsular India. Universities Press (India) Ltd, Hyderabad, India, xviii+254pp.
Kunte, K. (2011). Biogeographic origins and habitat use of the butterflies of the Western Ghats, south-western India. In: Priyadarshan, D.R., K.A. Subramanian, M.S. Devy & N.A. Aravind (eds.). Invertebrates in Western Ghats - Diversity and Conservation. Ashoka Trust for Research in Ecology and the Environment, Bengaluru.
Larsen, T.B. (1984). The zoogeographical composition and distribution of the Arabian butterflies. Journal of Biogeography 11: 119–158.
Larsen, T.B. (1987). The Butterflies of the Nilgiri Mountains of southern India (Lepidoptera: Rhopalocera). Journal of the Bombay Natural History Society 84: 291–316.
Niceville, L. de. (1890). The Butterflies of India, Burmah and Ceylon. Vol. III, Calcutta Central Press, Calcutta, xii+503pp.
Mahendroo, A. (2013). Talicada nyseus. Global Biodiversity Information Facility Data Portal accessed on 21 June 2013 (ID_parent/000472GBIF472969242)
Monsoon Report (2008). India Meteorological Department, National Climate Centre, Pune. Government of India, 197pp.
Monsoon Report (2009). India Meteorological Department, National Climate Centre, Pune. Government of India, 159pp.
Mott, C.L. (2010). Environmental Constraints to the Geographic Expansion of Plant and Animal Species. Nature Education Knowledge 3(10): 72.
Smetacek, P. (2009). Additions to the butterflies of Delhi. Bionotes 11(1): 15.
Smetacek, P. (2011). Four new lycaenid butterfly records from the Kumaon Himalaya, India. Journal of Threatened Taxa 3(2): 1555–1558; http://dx.doi.org/10.11609/JoTT.o2224.1555-8
Singh, A.P. (2005). Initial colonization of Red Pierrot butterfly, Talicada nyseus nyseus Guérin (Lycaenidae) in the lower western Himalayas: An indicator of the changing environment. Current Science 89(1): 41–42.
The Wealth of India, Vol. V, (1959). Council of Scientific and Industrial Research, New Delhi, xxv+332pp.
Wahlberg, N. (2006).That Awkward Age for Butterflies: Insights from the Age of the Butterfly Subfamily Nymphalinae (Lepidoptera: Nymphalidae). Systematic Biology 55(5): 703–714.
Wynter-Blyth, M.A. (1957). Butterflies of the Indian Region. Today & Tomorrow’s Printers and Publishers. New Delhi, xx+523pp.