Introduction
Vultures feed on carrion including discarded dead animals, which makes them an important component of the ecosystem. They also play an important cultural role in southern Asia (parts of India, Nepal and Tibet) as they consume human cadavers
which are left in the open during ritual sky-burials (Singh 1999; GON
2009; Liu et al. 2013). The decline of the vulture population in the Indian subcontinent has
removed a major scavenger population, with effects on other scavenging
species and the incidence of putrefying carcasses, both of which have
associated disease risks for wildlife, livestock and humans (GOI 2006).
India has nine species of vultures in the wild., viz.: Oriental White-backed (White-rumped) Vulture Gyps bengalensis, Slender-billed Vulture Gyps tenuirostris, Long-billed (Indian) Vulture Gyps indicus, Egyptian Vulture Neophron percnopterus, Red-headed (King) Vulture Sarcogyps calvus, Indian Griffon Vulture Gyps fulvus, Himalayan Griffon Gyps himalayensis, Cinereous Vulture Aegypius monachus and Bearded Vulture or Lammergeier Gypaetus barbatus (GOI 2006). Vultures
are known to inhabit tall trees in forests, smaller trees in open
areas, rocky cliffs, old monuments and the countryside (Thompson et al.
1990; Liberatori & Penteriani 2001; Donazar et al. 2002b; Carrete
& Donazar 2005; Monadjem & Garcelon 2005; Elorriaga et al. 2009; Thakur & Narang 2012; Harris
2013; Haenn et al. 2014). Except Griffons and Lammergeier, all Asian
vultures are in the threatened or Near Threatened categories (IUCN Red List 2011).
Vultures
are known to colonize wooded as well as open habitats with agriculture
and tree cover (Robinson 1994; Donazar et al. 2002a,b). On the basis of forest and vegetation cover, Uttar Pradesh (UP) has been divided into three major eco-zones: the Tarai (moist deciduous forests), the Gangetic plains (agriculture landscape) and the Bundelkhand (dry deciduous forests) including Vindhyan regions (Islam & Rahmani 2004). The file record of the UP Forest Department suggested that the western
part of the Gangetic plains, with a drier climate and ravined
landscape, is different from the main Gangetic plains and thus could be
categorized separately as a semi-arid zone. Knowledge
of ecological factors in the habitat affecting large scale distribution
and abundance of endangered species is an important tool for defining
management recommendations (Sutherland & Green 2004). Vultures inhabiting varying habitats have declined from many parts of
their former ranges owing mainly to food shortage and loss of habitat
(Satheesan 1999; Pain et al. 2003). Thus
the objective of this study is to assess species richness and
abundance, ecology and conservation issues related to vulture species in
varied eco-zones of UP and to keep the records for future reference.
Materials and Methods
Study site
Uttar Pradesh is one of the northern states of India with a tropical climate and a wide range of temperature fluctuation from 2–48 0C. There are three main seasons, summer from March to mid-June, rainy
season from mid-June to September and winter from October to February. There is great variation in rainfall from 600–2500 mm (Rahmani et al. 2011).
All the forest divisions (FD), the district level administrative units of the forest department of UP spread over the whole state (23052’–30024’N & 7705’–840 38’E) were involved in vulture survey and counting. A preliminary survey was done before the main counting which resulted
in the identification of FD where vultures were historically reported to
be seen. Sites
of vulture roosting and nesting were searched and marked on the basis
of physical and oral evidence in the respective FD during this survey
exercise. These potential vulture sites were revisited for vulture counting and other data collection.
Population estimation
Three
vulture counts were undertaken in different seasons: rainy season
(August 2010), summer (May 2011) and winter (December 2011). The population estimation protocol was revised and improved after every count. All the vultures seen were counted during the first estimation in different forest beats of Territorial and Social Forestry divisions
in the span of one week (second week of August 2010), assuming that
vultures would not have changed their roosting or nesting sites during
such a short period. In the second estimation a particular day was fixed (15 May
2011) for summer population estimation of vultures to avoid double
counting in case of movement of vultures to another territory.
Pre-estimation exercises were conducted to locate the roosting and
nesting sites in advance. This time the coordinates of vulture sites were recorded and an attempt was made to identify the vulture species. 15 December 2011
was fixed for the third or winter population estimation keeping in view
the arrival of migratory vultures during this period. An additional exercise of intensive identification of vulture species
was taken up during this estimation. For this a training workshop for
the trainers was conducted and an identification booklet (Jha et al.
2011) was provided. The number of unidentified vultures was distributed proportionately to the recorded species. Flying vultures were not included in the count in any of the three population estimation exercises.
A
team of two members, an observer and a data recorder, visited the
potential vulture sites on the designated day(s) based on the
pre-counting survey or the known area for vultures in all the vulture ranges (operational unit of a FD). Altogether
100 sites were visited by 58 members (29 teams) in the summer
estimation while 144 sites were covered by 84 personnel (42 teams) in
the winter estimation. They
counted the number of vultures (adult and juvenile) and nests, recorded
the details of roosting and nesting sites including geographical
location. Binoculars, GPS instrument and a predesigned format were used for data recording. Data were compiled forest division wise and then district wise.
Map generation
Thematic
maps providing roosting and nesting location of vulture population and
species distribution were prepared by using geographical locations. Arc GIS 9.3 was used for the conversion of GPS reading to spatial data
layer (point features providing the location). Vulture locations were
overlaid on the state map. Using the retrieval function various thematic
figures were designed.
Qualitative data
A
questionnaire survey was conducted about vulture ecology like, habitat
use, availability of trophic resources, other competitors for carrion,
conservation issues etc. among the forest guards and the villagers of
the FDs where vultures were recorded. Three-hundred-eighty-two completed questionnaires returned out of 500 circulated ones, 20 each in 25 vulture districts.
Some of the secondary data like cattle population, forest cover, and wild animals other than big cats, used in this article were adopted from UPFS (2010).
Results
The qualitative perception of the respondents (n=374) indicated that the vulture population had declined in the past 10–15
years and the main cause of this decline was use of diclofenac (n=277),
shortage of animal food (n=128) and habitat loss (n=117). The disposal of dead animals (n=296) was mainly carried out by throwing
away the carcass to the outskirts of the village, which attracted dogs,
crows and egrets that competed with vultures. They identified trees, monuments, cliffs, riversides, slaughter houses,
fertilizer factories and agriculture fields as vulture inhabiting
sites. The preferred trees for nesting and roosting in decreasing order were Sacred Fig Ficus religiosa > Banyan Ficus bengalensis > Silk Cotton Bombax ceiba > Sissoo Dalbergia sissoo. The prevalence of the vulture species as perceived by them in decreasing order was Egyptian Vulture > Indian Vulture > Slender-billed Vulture > Cinereous Vulture > others.
Population
The
minimum number of vultures seen in the state over three seasons: rainy
season, summer and winter during 2010 and 2011 were 2125, 2097 and 2029,
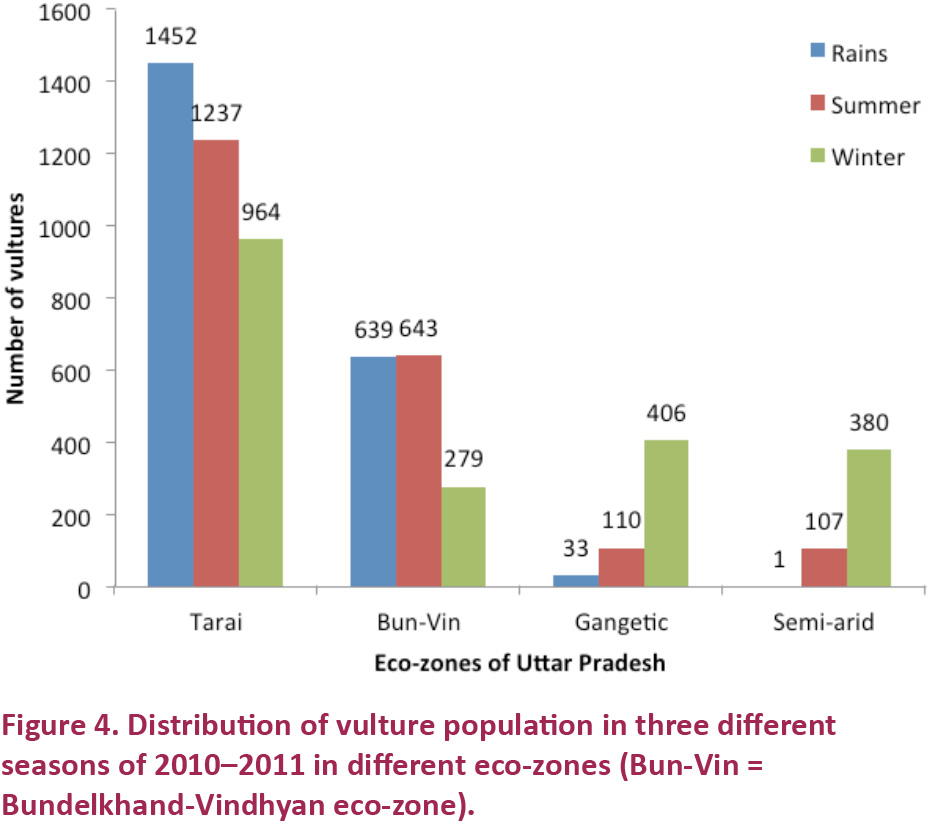
respectively (average 2084). Out of these vulture populations, juveniles were recorded at 4.2%, 4.0% and 4.5%, in respective seasons. Eco-zone distribution of total vultures in rainy season was 1452, 639,
33 and 1; in summer 1237, 643, 110 and 107; and in winter 964, 279, 406
and 380, respectively in Tarai, Bundelkhand-Vindhyan, Gangetic and semi-arid eco-zones. This showed that the bulk of the vulture population was confined to Tarai and Bundelkhand-Vindhyan eco-zones.
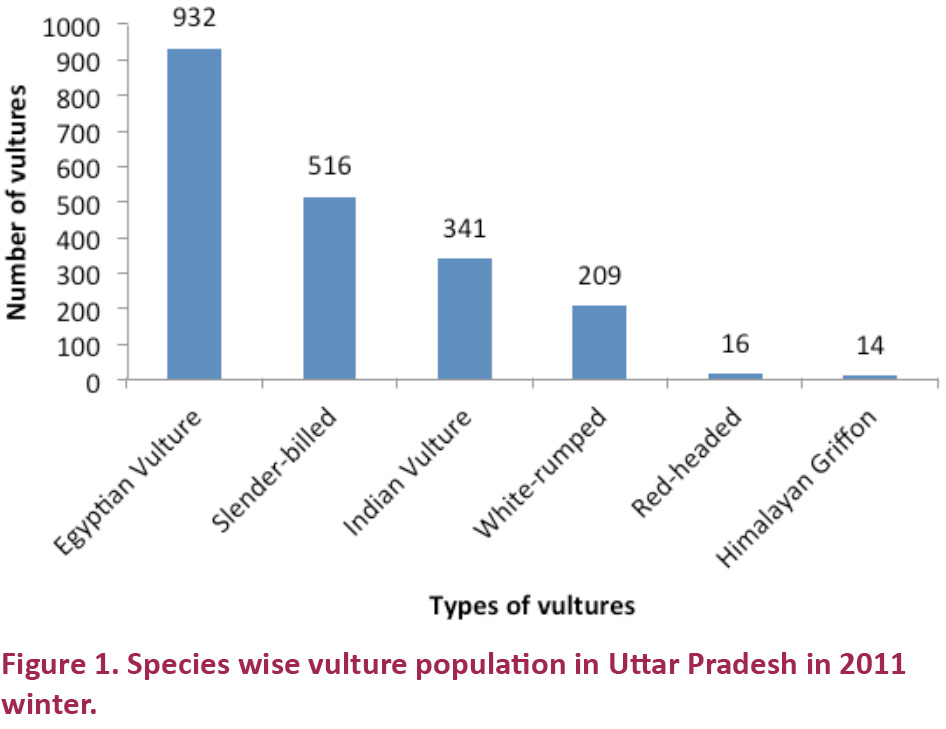
Altogether six vulture species were recorded in the state during the winter season. They
were Egyptian Vulture, Himalayan Griffon, Red-headed Vulture, Indian
Vulture, Slender-billed Vulture, and White-rumped Vulture. There was a report of one group of Cinereous Vulture, in Tarai region after a couple of days of the counting day (Image 1). Species
wise population distribution in order of decrease was Egyptian Vulture
> Slender-billed Vulture > Indian Vulture > White-rumped
Vulture > Red-headed Vulture > Himalayan Griffon (Fig. 1).

Distribution
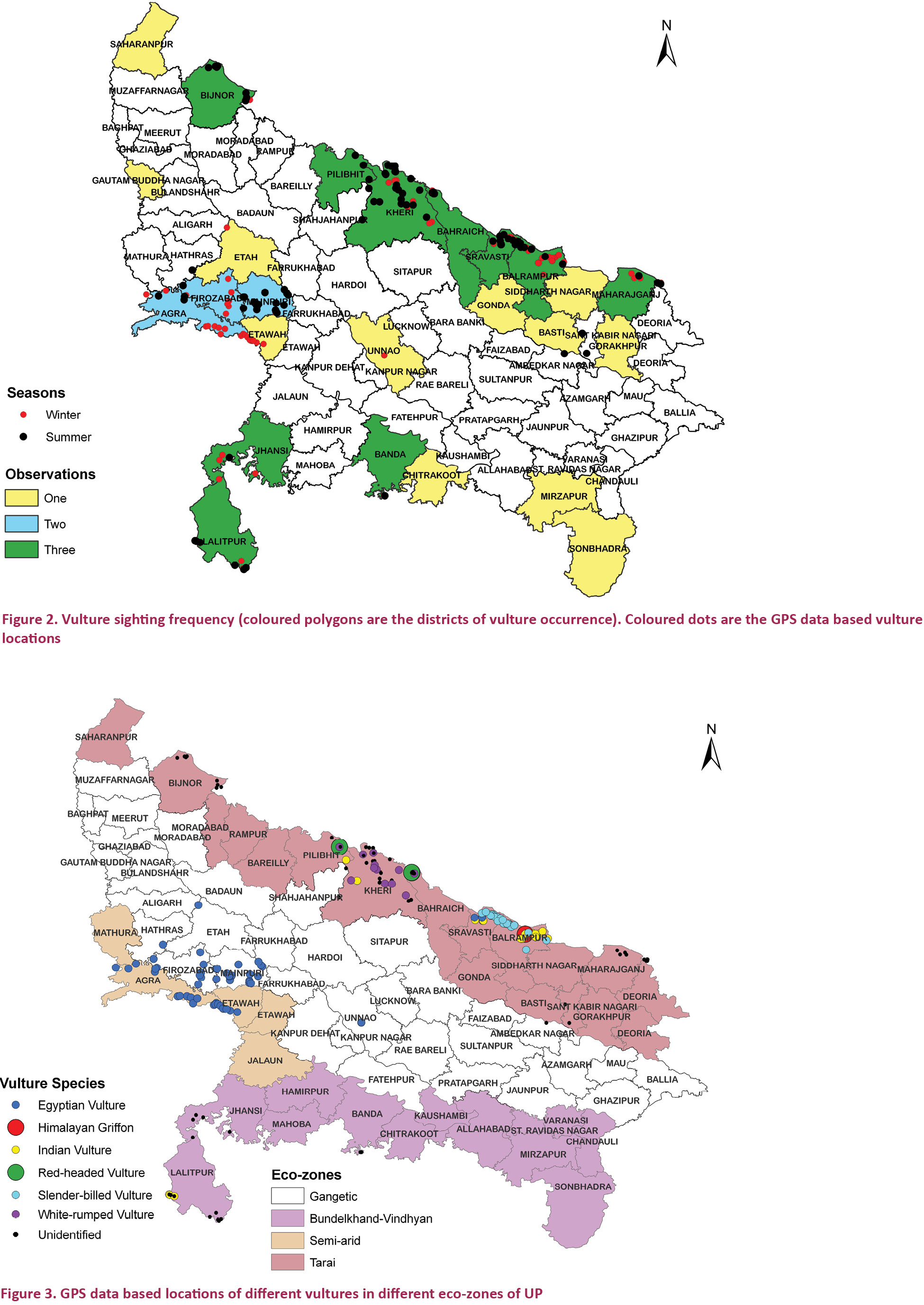
The vultures were represented in all the four eco-zones but were missing in many districts of the state. The
districts where vulture presence was recorded in all the three seasons
were Bahraich, Balrampur, Bijnore, Lakhimpur Khiri, Maharajganj,
Pilibhit, and Shravasti (Tarai eco-zone) and Banda, Jhansi and Lalitpur (Bundelkhand-Vindhyan eco-zone). When
considering vulture occurrence in at least one season, the additional
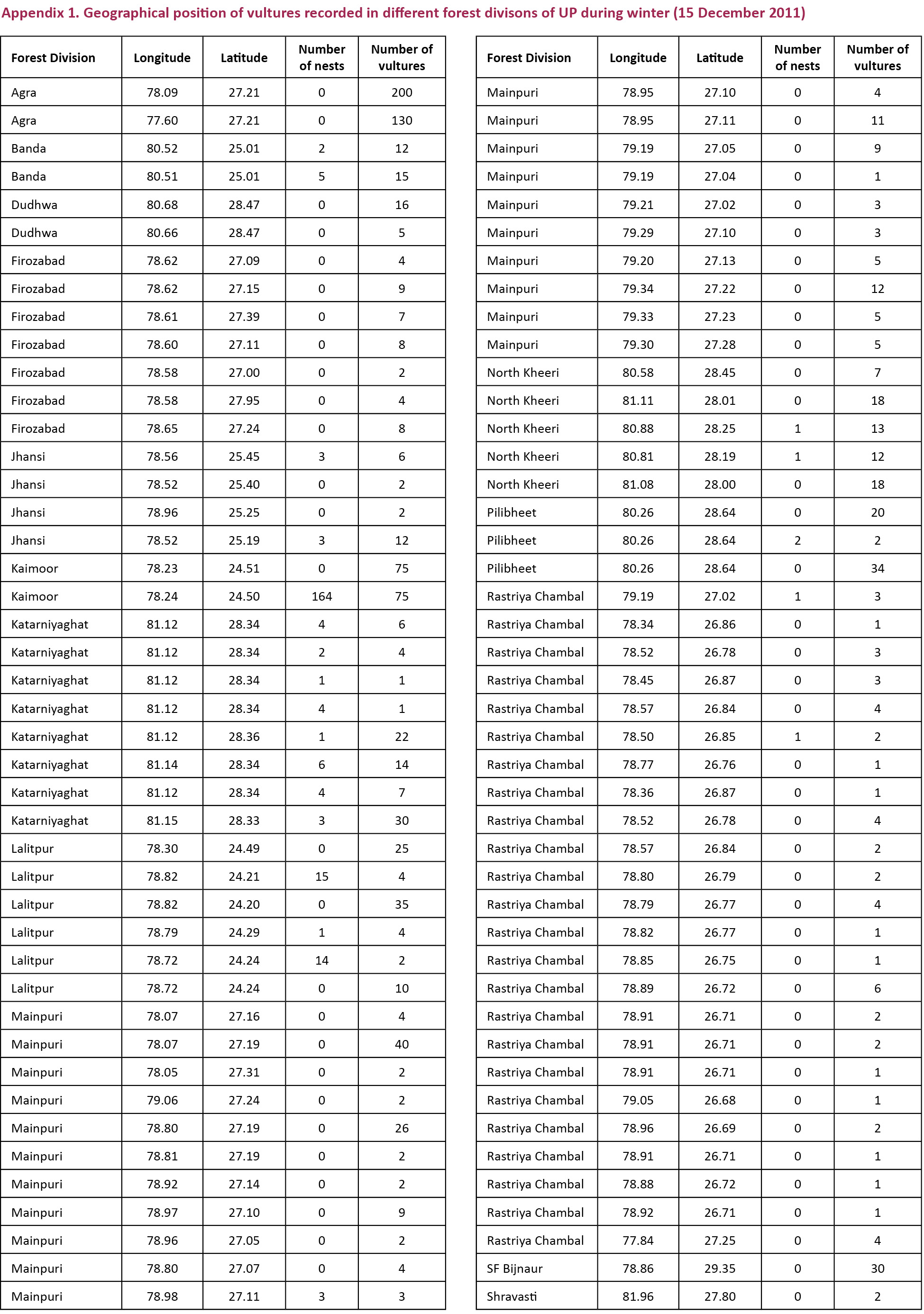
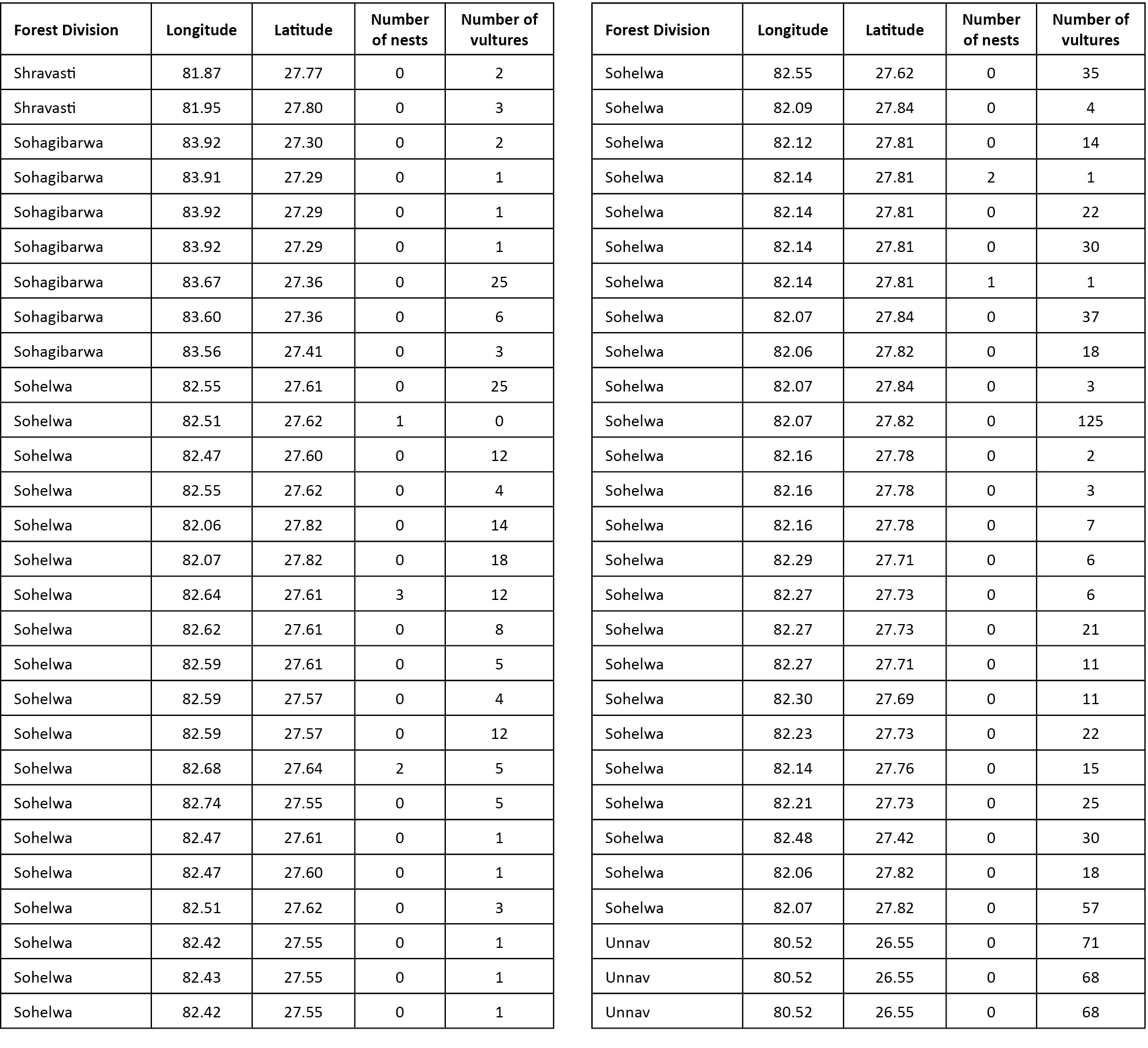
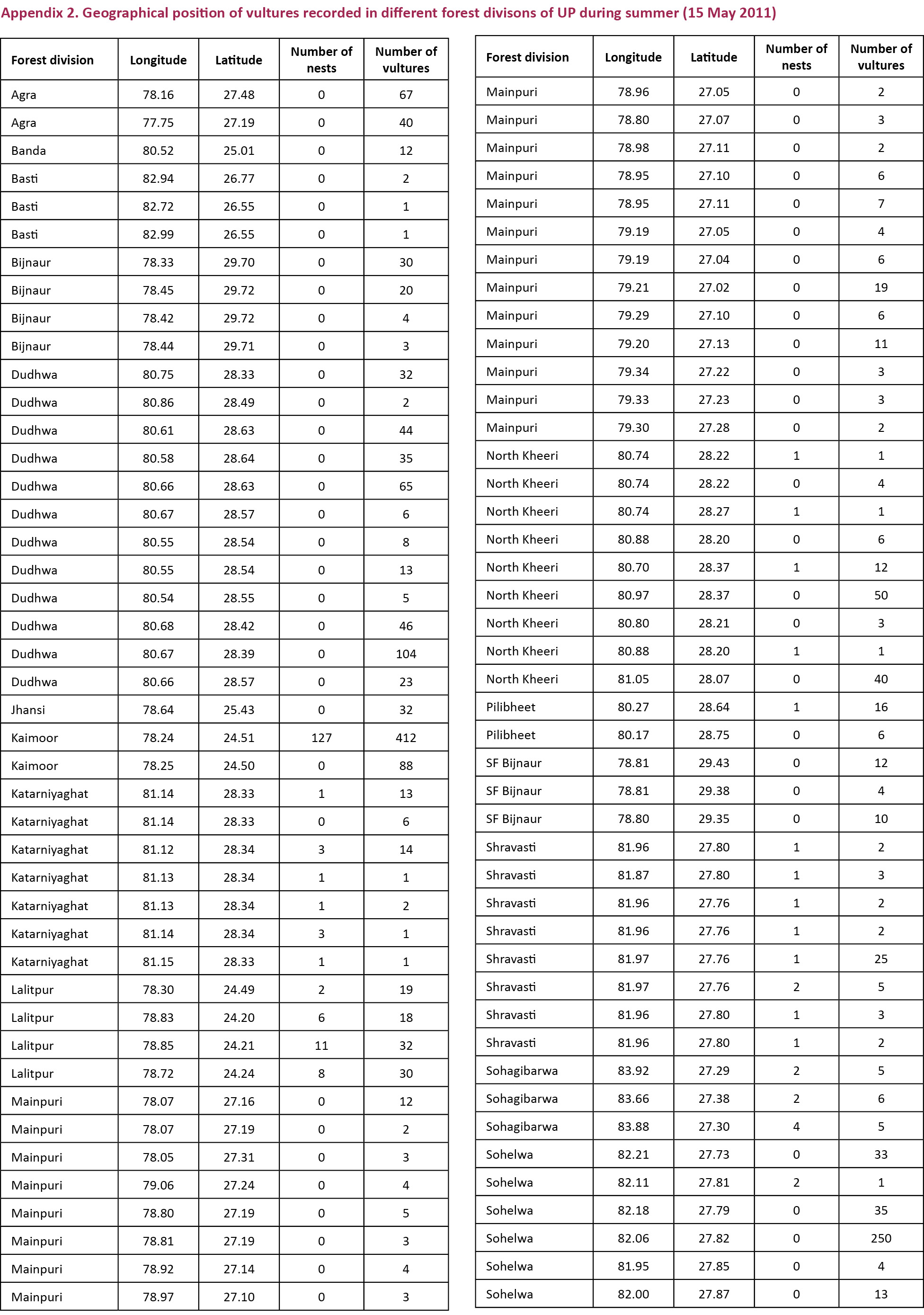
districts having vulture distribution were Agra and Etawah (semi-arid eco-zone), Basti, Gonda, Gorakhpur, Saharanpur and Sidharth Nagar (Tarai eco-zone), Chitrakoot, Mirzapur and Sonbhadra (Bundelkhand-Vindhyan eco-zone) and Etah, Firozabad, Gautambudh Nagar, Mainpuri, and Unnao (Gangetic eco-zone). Other districts in the state were devoid of vultures in all the three vulture counts conducted during 2010 and 2011 (Fig. 2). GPS location details of vultures provided by the field staff is given in Appendix 1 and 2.
The Tarai eco-zone
recorded highest number of vultures in all seasons and all of the six
species (Egyptian Vulture, Himalayan Griffon, Red-headed Vulture, Indian
Vulture, Slender-billed Vulture, and White-rumped Vulture) but the
Gangetic and Semi-arid eco-zones had only Egyptian Vulture and the Bundelkhand-Vindhyan region had Indian Vulture (Figs. 3 & 4). However, the concentration of all the vultures except Egyptian Vulture was very high in protected wildlife areas (national park and sanctuaries) as compared to the neighbouring forests.
Habitat use
Forest
area, scattered trees, rocky cliffs, monuments, open agriculture fields
and riversides were being used by different species of vultures for
nesting and roosting. Tree species used for nesting or roosting were Silk Cotton, Sissoo, Sacred Fig, Banyan, Haldu Haldina cordifolia, Axlewood Anogeisus latifolia, Cluster fig Ficus racemosa and Tamarind Tamarindus indica. Egyptian Vultures were seen on Cluster Fig and Tamarind, Himalayan Griffon on Sissoo, Indian Vulture on Sacred Fig, Slender-billed Vulture on Sissoo, Haldu, Axlewood and Sacred Fig and White-rumped Vulture on Sacred Fig and Silk Cotton trees (Image 2). Egyptian
Vultures were also seen resting on high tension line support frames and
wireless towers, and wandering in agriculture fields. Indian Vultures
were found using the ledges of rocky cliffs and the cornices of
monuments. White-rumped
Vultures were seen basking in the sun, some with necks drooping, along
the mud island of a river (Gerua in Katerniaght FD). Another colony of this species was seen roosting around a pair of nests on Silk Cotton tree in the proximity of a water body (at the bank of same river) and at the boundary of a hamlet. However, a vulture bathing in a water body was not recorded on the vulture counting dates. All the trees holding nests (5 Silk Cotton, 3 Sissoo, 2 Teak and 1 Haldu) and being used as roosting (4 Silk Cotton, 2 Sissoo, 1 Teak) visited during the summer and winter counting by the author, were tall (15–20 m) and with leafed crowns. In one case a Himalayan Griffon was seen roosting on top of a dry Sissoo tree. These trees were mostly old and matured. The presence of a large flock of Egyptian Vulture was recorded in and around the campus of a bone-based fertilizer factory.
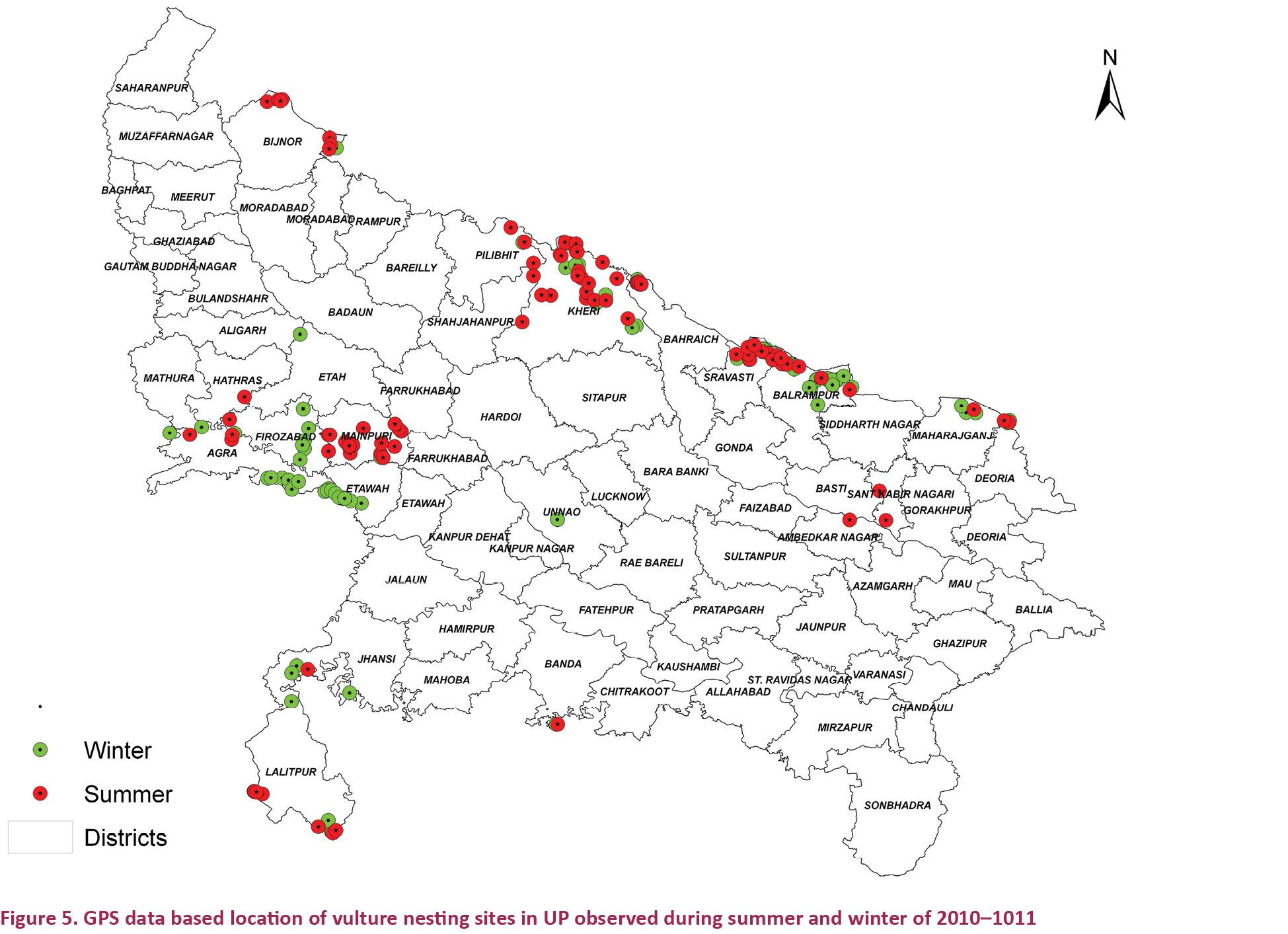
The
numbers of nests recorded during the summer and winter counts on the
basis of direct evidence were 73 and 75, respectively (Fig. 5). The vultures were either sitting inside the nests or were seen resting very close to them. These nests were mainly located in the Tarai eco-zone followed by the Bundelkhand-Vindhyan eco-zone. During the winter 2011 count 18 nests of White-rumped Vulture, seven nests of Red-headed Vulture, three nests of Slender-billed Vulture and five nests of Indian Vulture were seen in the Tarai eco-zone, while 37 nests of Indian vulture and six unidentified nests were seen in the Bundelkhand-Vindhyan eco-zone. One nest each of Egyptian Vulture was seen in the Gangetic and the semi-arid eco-zone. One
hundred twenty five additional nests were found empty but with faecal
deposits and some vegetation material in the cliffs of the Kaimoor
(Mahabir Swamy) Sanctuary (Bundelkhand-Vindhyan eco-zone). These
nests were assumed to be of Indian Vultures since this species was
recorded in large number (around 500 during summer count) in this
locality.

Discussion
The
risk of extinction greatly depends on the frequency of catastrophe,
survival rate and reduction in the population growth rate (Pavokovic
& Susic 2005). The total population figure of the state kept decreasing in subsequent counts. Although
data was too scanty for strong quantitative assessment (three temporal
populations of vultures in the span of 16 months) still, the apparently
negative growth of vulture population was corroborated with the
respondents’ observation that vulture population had decreased in the
past one and a half decade. The
decreasing population trend conformed to the previous reports (Prakash
1999; Gilbert et al. 2002; Prakash et al. 2003; Gilbert et al. 2006). But the rate of decrease in mixed population was slow (3.2%) as compared to a very high mortality rate (11–18 %) in the earlier report in select Gyps species (Khan & Murn 2011).
Kendall et al. (2014) suggested that prey mortality may be a more important driver of vulture habitat use than prey abundance. UP is a highly populated and agriculture dominated state, with poor
natural resource reserve and a livestock population of 64 million (UPFS
2010). The high livestock availability went in favour of vulture food security due to possible availability of carcasses and carrion. However, the situation on the ground was different, as informed by the
respondents, since most of the cattle were sold before they grew old.
This could be correlated to the fact that UP has a sizable beef eating
population and also a well-established leather industry (Kumar 2010). The
dead animals thrown at the outskirts of the village, by default
available food for vulture, had the probability of the presence of
diclofenac in the carrion as illegal diclofenac use was very common in
rural areas. This could cause trophic resource stress and may have a negative impact on vulture conservation in the state.
The Tarai and Bundelkhand-Vindhyan eco-zones
were the major vulture population centers, possibly due to higher
forest cover of which a large part is managed as wildlife conservation
reserve as compared to the Gangetic and the semi-arid eco-zones. In terms of vegetation Bundelkhand-Vindhyan, Tarai, Gangetic and semi-arid eco-zones
have 44, 35, 15, and 6% of total forest cover in the state,
respectively, compared to the geographical area 17, 25, 51 and 8 % of
the state (UPFS 2010). This suggested a decreasing density order of forests in Tarai, Bundelkhand-Vindhyan, Semi-arid and Gangetic eco-zones. The
occurrence of vultures in high vegetation areas was supported by
previous reports as vultures require a relict wooded area, mature trees
and a foraging range (Fargallo et al. 1998; Donazar et al. 2002a;
Carrete & Donazar 2005). They occur in protected forests (Donazar et al. 2002a; Monadjem &
Garecelon 2005; Sethi & Chauhan 2010; Das et al. 2011) and their
density was higher in the interface of protected and unprotected area
(Herremans & Herremans-Tonnocyr 2000 in Baral et al. 2005). As
compared to Tarai, Bundelkhand-Vindhyan zone did not have a very low
vegetation cover and yet very low number and types of vultures’
occurrence indicated towards a quality difference in habitat. Rocky
areas with dry deciduous vegetation in the region could be the reason
as also suggested by Bogliani et al. (2011) in the case of Bearded Vulture.
Forested districts provided both domestic as well as wild animals for food to the vultures. The Tarai and the Bundelkhand-Vindhyan eco-zones (4.3 and 2.1 animals km-2, respectively) had additional availability of food in the form of wild animals as compared to the Gangetic and the semi-arid eco-zones (3.0 and 2.7 animals km-2, respectively; UPFS 2010). Under
the prevailing circumstances of scarcity of domestic animal carcasses
due to their selling, availability of wild animals was an added
advantage for vulture presence as the dead bodies of wild animals, which
were diclofenac-free trophic resources, were available.
The Gangetic plain and the semi-arid eco-zones only had Egyptian Vultures. Food supply appears to be the limiting factor in these regions. The food supply available to any large scavenging animal came largely
from the carcasses of ungulates and these were widely dispersed,
transient and unpredictable in this location (Houston 2005). Domestic
ungulates were available outside forests but a majority of the cattle
were sold, resulting in non-availability of carcass as vulture feed, and
whatever was left from the transaction had the potential danger of
diclofenac infestation, possibly forcing the vultures to avoid feeding
on them. Egyptian Vultures could be seen in non-forested areas (semi-arid and Gangetic eco-zones) as they chose to feed on small animals, debris or rubbish dump, human and ungulate faeces, and vegetable matter (Whistler 1949; Prakash & Nanjappa 1988; Negro et
al. 2002) available in plenty, and lived in an open landscape in arid
and rugged areas (Liberatori & Penteriani 2001; Donazar 2002b). Their presence in large numbers in the campus of the bone factory indicated that they could feed on
bony remains of old dry carcasses collected for fertilizer making. A similar habit has been reported earlier in the case of Cape Vulture Gyps coprotheres (Vernon 2004).
Generally, lofty and sparsely branched trees in the forest area were used by large vultures for nesting and roosting. Such trees were helpful in providing safety from predators, a better view of surroundings and an easy take off (Yamac 2007). This also facilitated nocturnal perches with favourable microclimate by causing temperature inversion (Thompson et al. 1990). The use of large trees for nesting and roosting, including Silk cotton or Kapok or Semal, Sissoo, Teak, Haldu, Sacred Fig,
Banyan etc., had also been reported in different areas by other workers
(Baral et al. 2005; Satheesan & Khan 2005; Das et al. 2011; Kambale
2011). In the absence of tall trees, smaller trees in the open
landscape including Mango Mangifera indica, Babool or gum Arabic Accacia nilotica, Margosa Azadirachta indica (Kambale 2011) and Prosopis cineraria (Khatri 2013) were also reported to be used by the vultures in different areas. Taller trees were, however, dominant in number, 90% in the present case which is very similar to the 86% reported by Baral et al. (2005). The use of taller trees for nesting and roosting has been reported for
White-rumped Vulture (Thakur & Narang 2012) and White-backed Vulture Gyps africanus (Chomba & M’Simuko 2013).
It
was suggested that some vulture species had a tendency to choose dead
trees for roosting (Ceballos & Donazar 1990) which did not conform
to present findings. There is a report like the present one, where almost all the trees observed with vultures had leafed crowns (Yamac 2007). The
selection of artificial yet dangerous structure like electricity pylons
for roosting and nesting by some other vulture species had also been
reported earlier (van Rooyen 2004; Anderson & Hohne 2007; Chhangani
2009; Phipps et al. 2013).
The neck drooping posture in vulture was earlier recorded by some workers (Cunningham et al. 2003; Pain
et al. 2003; Prakash et al. 2003) and described as an indicator of
approaching death but contradicted later by Gilbert et al. (2007)
stating that it is an unsuccessful predictor of mortality and has a
probable role in thermoregulation. Until further reports sun basking, dozing off with a limping and
hanging neck appearance in White-rumped Vulture could be taken as a
normal activity of this species.
Management prescription and Conclusion
The
population of the vulture in UP was very low, especially the critically
endangered ones (Indian Vulture, Red-headed Vulture, Slender-billed
Vulture, White-rumped Vulture). Vulture
species in general are carrion feeders and old world vultures are slow
breeders with low growth rates (Donazar & Ceballos 1989). Therefore,
there is a need to take necessary steps to save them from all possible
threats, primarily by ensuring safe and sufficient food, recovery from
accidents and rehabilitation, and a protected environment.
Other
than diclofenac-tainted food, the most serious threat to vulture
species is the loss and alteration of habitat (Donazar et al. 2002a). Vultures are selective about tree species and individual trees for
nesting and roosting as they use tall trees with sparse but strong
branching. These
trees in the forest must be located, marked and protected because loss
of large trees in general would affect nesting negatively and in turn
the population (Monadjem & Garcelon 2005; Chomba & M’Simuko
2013). Since vultures are sensitive to disturbance during the breeding season
there should be an effort to maintain an anthropogenic disturbance free
zone around such nested and roosted trees. A buffer zone of a minimum of 500m (Margalida et al. 2010)
between source of disturbance and breeding colony should be used as
shock absorber, since minimum human disturbance is critical to
successful breeding of raptors (Bamford et al. 2009; Chomba &
M’Simuko 2013).
However, the base line data generated in this study could be used for future monitoring and further detailed study. Better supported findings could define better management action in the
direction of the conservation of this highly endangered group of
species.
References
Anderson, M.D. & P. Hohne (2007). African White-backed Vulture nesting on electricity pylons in the Kimberley area, northern Cape and Free state provinces, South Africa. Vulture News 57: 44–50.
Bamford, A.J., A. Monadjem & I.C.W. Hardy (2009). Nesting habitat preference of the African White-backed Vulture Gyps africanus and the effects of anthropogenic disturbance. Ibis 51–62; http://dx.doi.org/10.1111/j.1474-919X.2008.00878.x
Bogliani, G., R. Viterbi & M. Nicolino (2011). Habitat Use by a Reintroduced Population of Bearded Vultures (Gypaetus barbatus) in the Italian Alps. Journal of Raptor Research 45(1): 56–62; http://dx.doi.org/10.3356/JRR-09-69.1
Baral, N., R. Gautam & B. Tamang (2005). Population status and breeding ecology of White-rumped Vulture Gyps bengalensis in Rampur valley, Nepal. Forktail 21: 87–91.
Carrete, M. & J.A. Donazar (2005). Application of central-place foraging theory shows the importance of
Mediterranean dehesas for the conservation of the Cinereous Vulture Aegypus monachus. Biological Conservation 126: 582–590.
Ceballos, O. & J.A. Donazar (1990). Roost tree characteristics, food habits and seasonal abundance of roosting Egyptian Vultures in northern Spain. Journal of Raptor Research 24: 19–25.
Chhangani, A.K. (2009). Present status of vultures in the great Indian Thar Desert, pp. 65–83. In Sivaperuman, C. (ed). Faunal Ecology and Conservation of the Great Indian Desert. Heidelberg: Springer Verlag Berlin.
Chomba, C. & E. M’Simuko (2013). Nesting patterns of raptors; White-backed Vulture (Gyps africanus) and African Fish Eagle (Haliaeetus vocifer), in Lochinvar National Park on the kafue flats, Zambia. Open Journal of Ecology 3(5): 325–330; http://dx.doi.org/10.4236/oje.2013.35037
Cunningham,
A.A., V. Prakash, D.J. Pain, G.R. Ghalsasi, A.H. Wells, G.N. Kolte, P.
Nighot, M.S. Goudar, S. Kshirsagar & A. Rahmani (2003). Indian vultures: victims of a disease epidemic? Animal Conservation 6: 189–197.
Das, S.K., A. Dashahare, S. Marathe, N. Kundu & R. Kesharwani (2011). Status of raptors with special reference to vultures in and around Rajaji National Park, India. World Journal of Zoology 6: 350–356.
Donazar, J.A. & O. Ceballos (1989). Growth rates of nestling Egyptian Vultures Nephron percnopterus in relation to brood size, hatching order and environmental factors. Ardea 77: 217–226.
Donazar, J.A., G. Blanco, F. Hiraldo, E. Soto-Largo & J. Oria (2002a).Effects of forestry and other land-use practices on conservation of Cinereous vultures. Ecological Applications 12: 1445–1456.
Donazar, J.A., C.J. Palacio, L. Gangoso, O. Ceballos, M.J. Gonzalez, & F. Hiraldo (2002b). Conservation status and limiting factors in the endangered population of Egyptian Vulture (Neophron percnopterus) in the Canary Islands. Biological Conservation 107: 89–97.
Elorriaga, J., I. Zuberogoitia, I. Castilo, A. Azkona, S. Hidalgo, L. Astorkia, F. Ruiz-Moneo & A. Iraeta (2009). First documented case of long distance dispersal in the Egyptian Vulture. Journal of Raptor Research 43: 142–145.
Fargallo, J., G. Blanko & E. Soto-Largo (1998). Forest management effects on nesting habitat selected by Eurasian Black Vulture (Aegypus monachus) in central Spain. Journal of Raptor Research 32(3): 202–207.
Gilbert,
M., M.Z. Virani, R.T. Watson, J.L. Oaks, P.C. Benson, A.A. Khan, S.
Ahmad, J. Chaudhry, M. Arshad, S. Mahmood & Q.A. Shah (2002). Breeding and mortality of Oriental White-backed Vulture Gyps bengalensis in Punjab province. Bird Conservation International 12: 311–326.
Gilbert,
M., R.T. Watson, M.Z. Virani, J.L. Oaks, S. Ahmed, M.J.I. Chaudhary, M.
Arshad, S. Mahmood, A. Ali & A.A. Khan (2006). Rapid population declines and mortality cluster in three Oriental White-backed Vulture Gyps bengalensis colonies due to diclophenac poisoning. Oryx 40: 388–399.
Gilbert,
M., R.T. Watson, M.Z. Virani, J.L. Oaks, S. Ahmed, M.J.I. Chaudhary, M.
Arshad, S. Mahmood, A. Ali & A.A. Khan (2007). Neck-drooping posture in oriental White-backed Vultures (Gyps bengalensis): An unsuccessful predictor of mortality and its probable role in thermoregulation. Journal of Raptor Research 41: 35–40.
GOI (2006). Action Plan for Vulture Conservation in India. New Delhi: Ministry of Environment and Forests.
GON (2009). Vulture Conservation Action Plan for Nepal (2009–2013). Kathmandu: Department of National Parks and Wildlife Conservation, Ministry of Forests and Soil Conservation.
Haenn, N., B. Schmook, Y.M. Reyes & S. Calmé (2014). A cultural consensus regarding the king vulture?: preliminary findings and their application to Mexican conservation. Ethnobiology and Conservation 3: 1 (12 June 2014); http://dx.doi.org/10.15451/ec2014-1-3.1-1-15
Harris, R.J. (2013). The conservation of Accipitridae vultures of Nepal: a review. Journal of Threatened Taxa 5(2): 3603–3619; http://dx.doi.org/10.11609/JoTT.o2816.3603-19
Houston, D.C. (2005). Reintroduction programmes for vulture species. Conservation and
management of vulture population. Thessaloniki, Greece: Natural history
museum of Crete, Greece, 87–97pp.
Islam, M.Z. & A.R. Rahmani (2004). Important Bird Areas in India: Priority Sites for Conservation. Bombay Natural History Society, Mumbai.
IUCN (2011). The IUCN red list of threatened species. http://www.iucnredlist.org/apps/redlist/search.
Jha, K.K., P. Singh, A. Kanaujia & S. Kushwaha (2011). Vulture Species in Uttar Pradesh: An identification key. Lucknow,
India: Forest Department, UP and Zoology Department, Lucknow University.
Kambale, A.A. (2011). A study on breeding behaviour of oriental White-backed Vulture (Gyps bengalensis) in Anjarle and Deobag, Maharashtra. Wildlife Institute of India, Dehradun.
Kendall, C.J., M.Z. Virani, J.G.C. Hopcraft, K.L. Bildstein & D.I. Rubenstein (2014). African vultures don’t follow migratory herds: scavenger habitat use is not mediated by prey abundance. PLoS ONE 9(1): e83470; http://dx.doi.org/10.1371/journal.pone.0083470
Khan, U. & C. Murn (2011). Gyps vulture restoration project - Role of captive breeding in endangered species management. The Journal of Animal and Plant Sciences 21: 405–409.
Khatri, P.C. (2013). Home range use of migratory vultures in and around Jorbeer, Bikaner (Rajasthan) India. Bioscience Discovery 4(1): 96–99.
Kumar, N.P. (2010). Uttar Pradesh’s manufacturing sector: State, structure and performance. Internet article. Accessed on 30/10/2014 <www.telanganastat.com/article/23/nomita/fulltext.pdf>.
Liberatori, F. & V. Penteriani (2001). A long-term analysis of declining population of the Egyptian Vulture in Italian peninsula: distribution, habitat preference, productivity and conservation implications. Biological Conservation 101: 381–389.
Liu, C., Z. Huo & X. Yu (2013). Population and conservation status of the Himalayan Griffon (Gyps himalayensis) at the Drigung Thel Monastery, Tibet, China. Chinese Birds 4(4): 328–331.
Margalida, A., R. Moreno-Opo, B.E. Arroyo & A. Arredondo (2010). Reconciling the conservation of endangered species with economically
important anthropogenic activities: interaction between cork
exploitation and the cinereous vulture in Spain. Animal Conservation 1–8.
Monadjem, A. & D.K. Garcelon (2005). Nesting distribution of vultures in relation to land use in Swaziland. Biodiversity and Conservation 14: 2079–2093; http://dx.doi.org/10.1007/s10531-004-4358-9
Negro,
J.J., J.M. Grande, J.L. Tella, J. Garido, D. Homero, J.A. Donazar, J.A.
Sanchez-Zapata, J.R. Benitez & M. Barcell (2002). An unusual source of essential carotenoid. Nature 47(6883): 807.
Pain,
D.J., A.A. Cunningham, P.F. Donald, J.W. Duckworth, D.C. Houstan, T.
Katzner, J. Pary-Jones, C. Poole, V. Prakash, P. Round & R. Timmins
(2003). Causes and effects of temporospatial declines of Gyps vultures in Asia.Conservation Biology 17: 661–671.
Pavokovic, G. & G. Susic (2005).Population viability analysis of (Eurasian) Griffon Vulture Gyps fulvus in Croatia. Conservation and management of Vulture populations. Thessaloniki, Greece: Natural History Museum of Crete, WWF Greece, 75–86pp.
Phipps, W.L., K. Wolter, M.D. Michael, L.M. MacTavis & R.W. Yarnell (2013). Do powerlines and proteted areas present a catch - 22 situation for Cape Vultures (Gyps caprotheres)? PLos ONE 8(10): e76794; http://dx.doi.org/10.137/journal.pone.0076794
Prakash, V. (1999). Status of Vultures in Keoladeo National Park, Bharatpur, Rajasthan with special reference to population crash in Gyps species. Journal of the Bombay Natural History Society 96: 365–378.
Prakash, V. & C. Nanjappa (1988). An instance of active predation by Scavenger Vulture (Neophron p. gingianus) on Checkered-keelback Watersnake (Xenochrophis piscator) in Keoladeo National Park, Bharatpur, Rajsthan. Journal of the Bombay Natural History Society 85: 419.
Prakash, V., D.J. Pain, A.A. Cunningham, P.F. Donald, N. Prakash, A. Varma, R. Gargi, S. Sivakumar & A.R. Rahmani (2003). Catastrophic collapse of Indian White-backed Gyps bengalensis and Long-billed Gyps indicus vulture population. Biological Conservation 109: 381–390.
Rahmani, A.R., M.Z. Islam, V.P. Singh & S. Chaudhary (2011). Important Bird Areas of Uttar Pradesh: Priority sites for conservation. Katerniaghat Foundation, Lucknow.
Robinson, S.K. (1994). Habitat selection and foraging ecology of raptors in Amazonian Peru. Biotropica 26: 443–458.
Satheesan, S.M. (1999). The decline of vultues in India. Vulture News 40: 35–36.
Satheesan, S.M. & S. Khan (2005). Vulture paradise in the Katerniaghat Wildlife Sanctuary,
Uttar Pradesh, India. Conservation and Management of Vulture
Population. Natural History Museum of Crete & WWF Greece, Thessaloniki, Greece, p. 168.
Sethi, J. & N.P. Chauhan (2010). Nesting of rare vultures in Katerniaghat Wildlife Sanctuary, Uttar Pradesh, India. Indian Forester 136: 1372–1375.
Singh, R.B. (1999). Ecological strategy to prevent vulture menace to aircraft in India. Defence Science Journal 49: 117–121.
Sutherland, W.J. & R.E. Green (2004). Habitat assessment, pp. 251–268. In: Sutherland, W.J., I. Newton & R.E. Green (eds.). Bird Ecology and Conservation Oxford. Oxford University Press.
Thakur, M.L. & S.K. Narang (2012). Population status and habitat-use pattern of Indian White-backed Vulture (Gyps bengalensis) in Himachal Pradesh, India. Journal of Ecology and the Natural Environment 4(7): 173–180.
Thompson, W.L., R.H. Yahner & L.S. Gerald (1990). Winter use and habitat characteristics of vulture communal roosts. The Journal of Wildlife Management 54(1): 77–83.
UPFS (2010). Uttar Pradesh Forest Statistics. Forest Department Uttar Pradesh, Lucknow.
van Rooyen, C. (2004). Report on vulture interactions with powerlines in southern Africa, pp. 182–194. In: Monadjem, A., M.D. Anderson, S.E. Piper & A.F. Boshoff (eds). Vultures in The Vultures of Southern Africa - Quo Vadis? Proceedings
of a workshop on vulture research and conservation in southern Africa.
Birds of Prey Working Group, Johannesburg. McGregor Museum, Kimberly, South Africa.
Vernon, C. (2004). The Eastern Cape’s Vultures, pp. 78–80. In: Monadjem, A., M.D. Anderson, S.E. Piper & A.F. Boshoff (eds). Vultures in The Vultures of Southern Africa - Quo Vadis? Proceedings of a workshop on vulture research and conservation in southern Africa. Birds of Prey Working Group, Johannesburg. Kimberly, South Africa: McGregor Museum.
Whistler, H. (1949). Popular Handbook of Indian Birds. Gurney and Jackson, London.
Yamac, E. (2007). Roosting tree selection of Cinereous Vulture Aegypius monachus in breeding season in Turkey. Podoes 2: 30–36.