INTRODUCTION
The reproductive biology of bats is relatively under studied. Mating systems and post-natal development of juvenile bats are the two topics that have been most commonly investigated. Bats represent an extremely diverse and abundant group of organisms, with 1304 species having been described to date (Nancy Simmons, pers. comm. 2014). Consequently, they exhibit a wide variety of mating systems, which are either monogamous or polygynous and occur seasonally or exist on a permanent, year-round basis (McCracken & Wilkinson 2000). Lek (Bradbury 1977), harem (Kunz et al. 1983), promiscuous (Thomas et al. 1979; Keeley & Keeley 2004), polygynous (Rossiter et al. 2000), and monogamous (Vaughan & Vaughan 1987) mating systems have all been described in bats. Complementing this body of research are numerous studies assessing neonatal development, which primarily track the growth of juveniles by measuring changes in weight and forearm length over time (Kunz & Robson 1995; Hoying & Kunz 1998) and/or attempt to quantify the effect of abiotic environmental factors, such as temperature and precipitation rate, on development through the construction of growth curves (Hoying & Kunz 1998; Hood et al. 2002). Additionally, biologists have developed standardized categories to measure levels of development at birth (i.e., altricial, precocial, or intermediate), based on a number of species-specific characteristics ranging from maternal size and average number of young per litter to the length of gestation and neonate eye-opening time (Kurta & Kunz 1987; Derrickson 1992).
In contrast, little is known about parturition in bats. Of the parturition studies that have been conducted, few have described the entire process for a species based on observations made on wild animals in their natural environment. Furthermore, the majority of such studies are not contemporary, having been carried out in the late 1800s and early to mid-1900s (Blake 1885; Sherman 1937; Wimsatt 1945; Jones 1946; Ramakrishna 1950; Nelson 1965); sometimes they were just isolated accounts written by curious amateur naturalists (Blake 1885). Moreover, most of these observations have relied on data collected from captive animals (usually fewer than three individuals) housed in artificial environments (Jones 1946; Bogan 1972; Easterla 1976; Kurta & Stewart 1990). And oftentimes, biologists seek only to describe a single behavior rather than provide a complete characterization of the entire birthing process itself (Webb et al. 1992; Kunz et al. 1994).
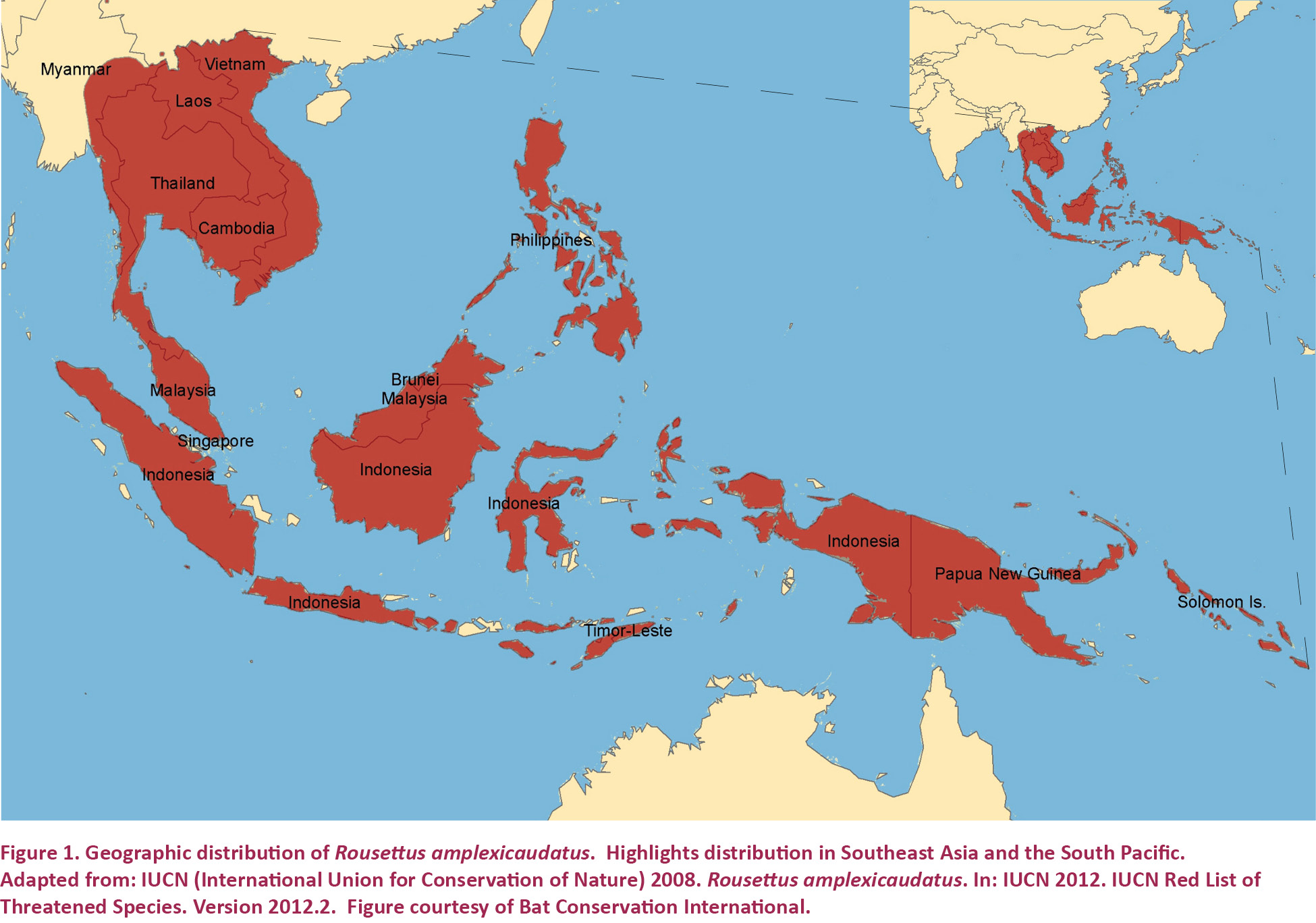
Almost no research has been conducted on the reproductive biology of Geoffroy’s Rousette Fruit Bat Rousettus amplexicaudatus, a frugivorous Yinpterochiropteran (Bergmans 1997; Springer et al. 2001). Rousettus amplexicaudatus is native to Southeast Asia and the South Pacific, with populations found from Vietnam and Myanmar to Papua New Guinea and the Solomon Islands (Fig. 1) (Simmons 2005). As far as we can determine, only two reproductive studies have been carried out on this species; analysis of parturition was the goal of neither. Instead, the first study characterized fetal development of lips, patagia, and other key morphological structures associated with the integumentary system (Giannini et al. 2006), while the second assessed seasonal variation in the timing of reproductive events of populations inhabiting the central Philippines (Heidiman & Utzurrum 2003). To our knowledge, complete observations on the natural parturition process of this species have never before been published. Consequently, our understanding of parturition in R. amplexicaudatus is fragmented and incomplete and has been assembled using pieces of data that were primarily collected as the inadvertent byproducts of other studies. Past research indicates that R. amplexicaudatus gives birth after a three to four month gestation period (Heideman & Utzurrum 2003). Furthermore, it is thought that females have bimodal estrous, which means that young are typically produced twice annually (Heideman & Utzurrum 2003). Additionally, we believe that R. amplexicaudatus is a monotocous species, as polytocous females have never before been documented. Outside these few details, little else is known about the parturition process of this species.
The general lack of information regarding reproductive bat biology can be explained by the lifestyles exhibited by these organisms. The basic biology of bats makes studying them challenging in nearly all aspects. To do so, researchers must carefully consider time, location, equipment, and safety. As nocturnal mammals, bats are primarily active when humans are not. And as volant animals, bats are able to disperse large distances. This makes it extremely difficult for researchers to track their movement. Furthermore, bats tend to inhabit places that can be remote, difficult to access, and/or hazardous to explore (e.g., crevices in tall cliffs, deep underground caves, abandoned mines, and decommissioned military bunkers). Cave-dwelling bats, such as R. amplexicaudatus, tend to move away from the lighted areas near the openings of caves and roost in spaces where little ambient light is present (Twente 1955). Presumably this helps them hide from predators and find microclimates that are suited to their specific biological needs (Baudinette et al. 2000). However, it is difficult for biologists to study bats under these conditions, as the use of artificial light (typically required to observe bats in these underground environments) may have a direct adverse impact on the animals’ behavior (Downs et al. 2003; Stone et al. 2009), making it impossible to study their natural behavior.
Our lack of knowledge regarding the specific details of the reproductive biology of R. amplexicaudatus results from an additional set of circumstances. Presently, R. amplexicaudatus is listed by the International Union for Conservation of Nature (IUCN) as a species of Least Concern and biologists have documented multiple large roosts of this species (Csorba et al. 2008). Because it occurs in vast numbers across an extensive geographic range, R. amplexicaudatus is neither a uniquely endemic species nor an archetype for endangered animal conservation, which makes it an unpopular candidate for research. Unfortunately, this serves to restrict opportunities to expand our knowledge of its life history and behavioral ecology.
This study will help improve our understanding of R. amplexicaudatus by describing the behavioral sequence associated with parturition in a wild colony inhabiting a cave on the Island Garden City of Samal (Samal Island) in the southernmost region of the Philippines. It is just one part of a larger research project that was conducted at this field site, which aims to observe, analyze, and describe various aspects of the behavior and ecology of this species. This particular field site provided the ideal location for a non-invasive research project. The cave has many entrances where the bats roost in broad daylight, which leaves them visible from outside of the cave and allowed us to observe their behavior and social interactions without having to enter the cave and disturb its inhabitants. Furthermore, the cave is inhabited by the largest recorded colony of R. amplexicaudatus, approximately 1.8 million bats (Carpenter et al. 2014). With an estimated density of 422.3 bats per square meter (Carpenter et al. 2014), the cave provided abundant opportunities for observation under optimal conditions.
MATERIALS AND METHODS
Data were collected from January 17 to February 5 and July 11 to October 9, 2011. The field site, Monfort Bat Cave, is located on Samal Island, Davao del Norte, Mindanao, Philippines. The average temperature of this region is 26.60C, with relative humidity ranging from 71–85 %. Generally, two climatic seasons are recognized in the Philippines: dry (December to May) and rainy (June to November). However, the Philippine Atmospheric Geophysical and Astronomical Services Administration divides the country into four climate types. Samal Island is classified as “Climate Type IV,” indicating that is has no defined dry season and even annual rainfall patterns. Interestingly, typhoons have a minimal impact on the southern Philippines. The site of origin and typical trajectory of these weather systems cause them to miss the Mindanao region, which makes the area an ideal setting for much of the country’s industrial, agricultural, and tourism sectors (Manalo & Cinco 2011). Thus, it is not surprising that commercial and subsistence fishing, recreation, and tourism are the main industries present on Samal Island (Ness 2003).
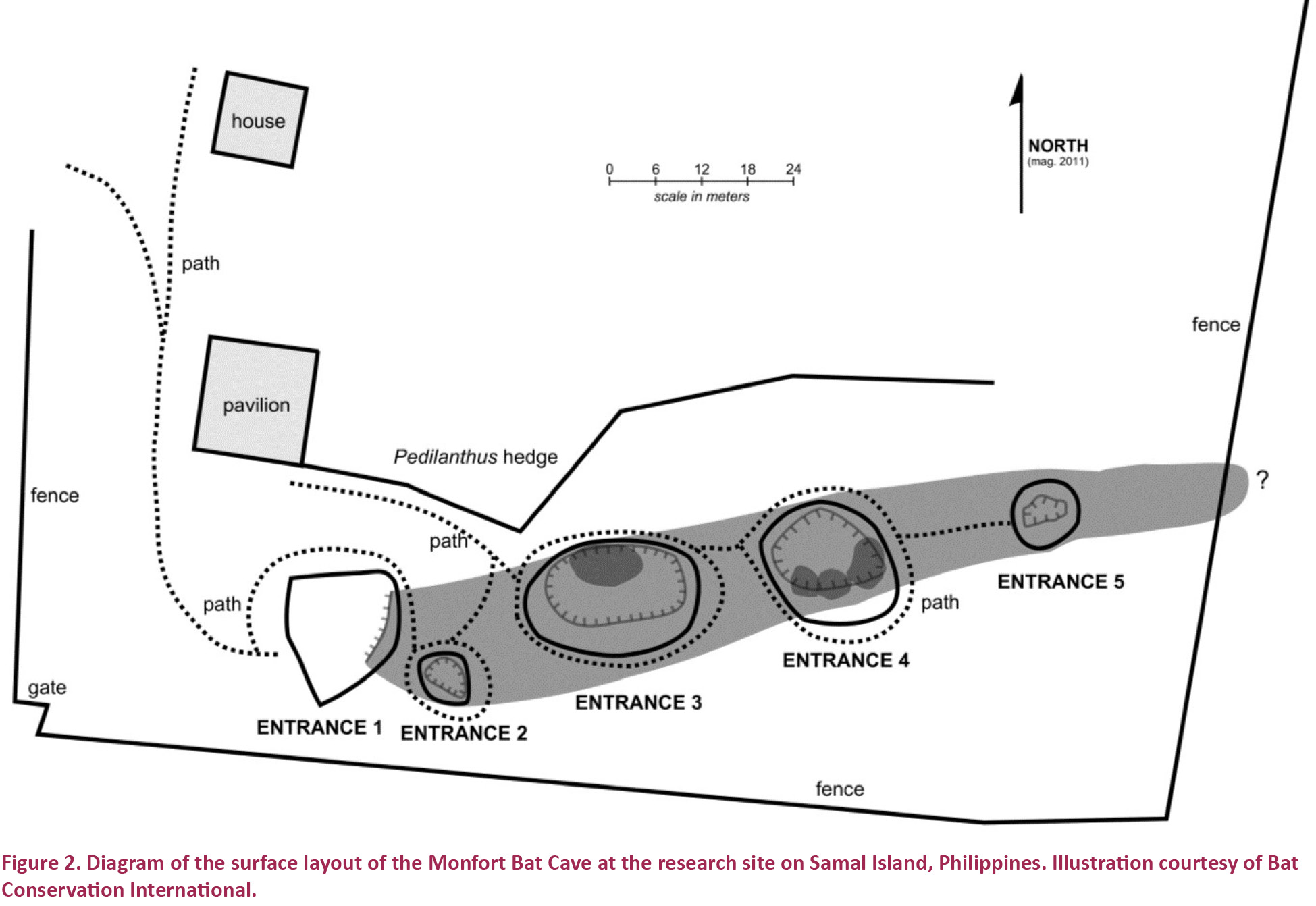
Monfort Bat Cave consists of a single chamber that is approximately 150m in length (Carpenter et al. 2014); the cave interior has not been formally mapped and surveyed, leaving more precise internal measurements pending. The cave opens to the surface in five sequential entrances (Fig. 2). It is located less than 200m from the Gulf of Davao (Carpenter et al. 2014), on a hillside that slopes upward from the coastline. The cave is oriented in such a way that the first entrance is at a lower elevation than the rest and each subsequent entrance is at a slightly higher elevation than the one preceding it. The first entrance of the cave is horizontal, while the remaining four entrances are vertical. The cave, inhabited by the world’s largest known colony of R. amplexicaudatus (approximately 1.8 million bats), operates as a sanctuary and is open for public visitation on a daily basis. Guests receive guided tours around the outside of the cave, but are not allowed access to its interior. Consequently, each entrance is protected by a pole fence as a safety and security precaution, as can be seen in Fig. 2.
Handheld, high definition video cameras were mounted on tripods outside the cave’s perimeter fences and used to record bat activity. We filmed at all five entrances of the cave. A 360 degree field of view was available for each of the vertical openings (entrances 2–5). The vegetation surrounding the cave, which consisted primarily of coconut palms, did not significantly affect our visibility at any of the entrances. Video was recorded only during the daylight hours when bats were actively roosting in the cave. Our data collection activities were suspended each afternoon when the video quality on the cameras’ LCD screens degraded enough that the human eye could no longer easily distinguish individual bats from one another. Sampling was also suspended during periods of heavy rainfall. Focal animal sampling, conducted only during the daytime, was used to collect parturition data. Observations were made on individual pregnant bats and their behavior was sampled continuously throughout the parturition process, for as long as the females remained visible. None of the observations were made across multiple days of sampling. As such, no effort was made to identify and track individual bats across time and space.
OBSERVATIONS
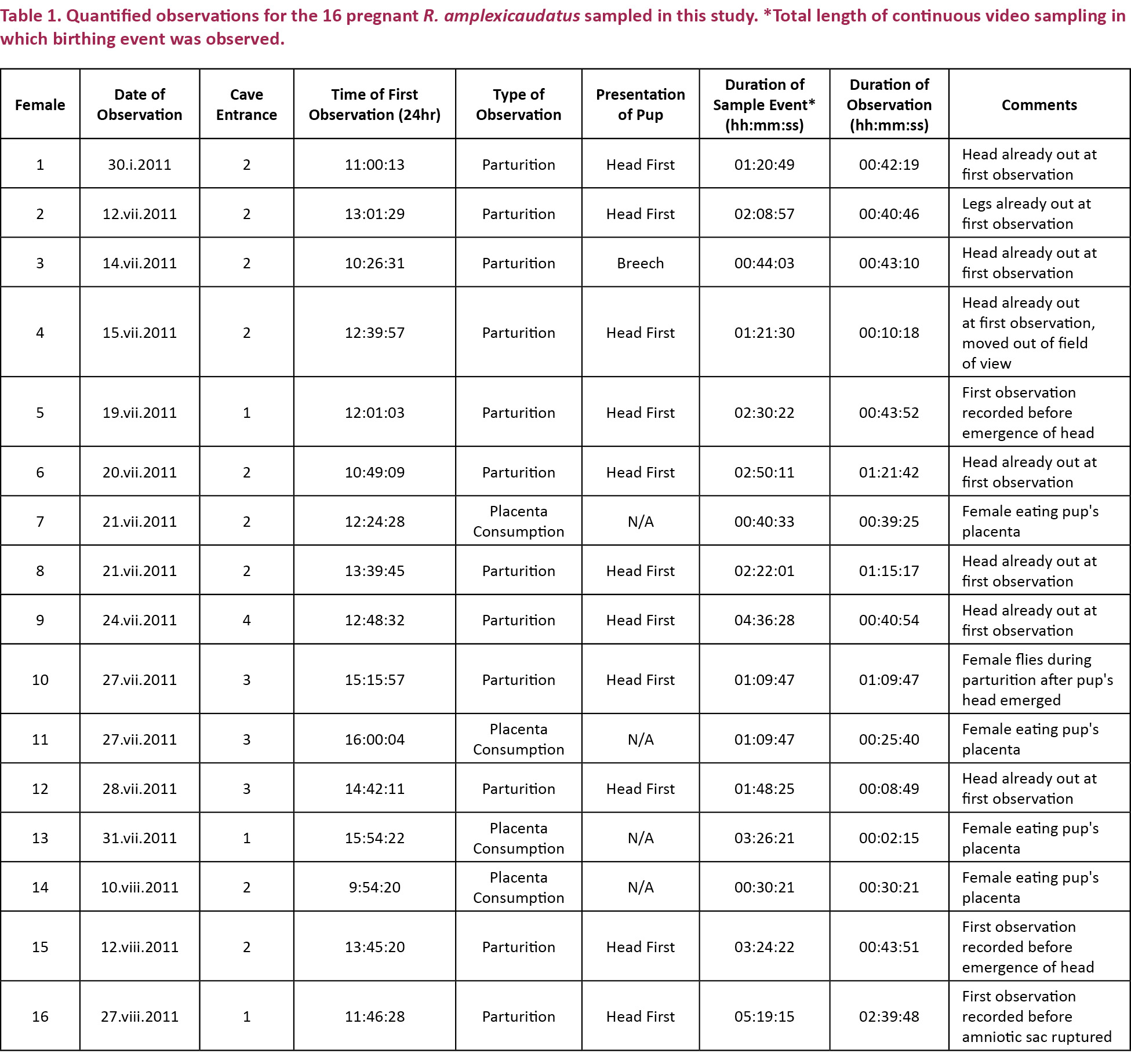
Approximately 330hr of video was recorded overall; an average of 30hr of video was recorded per week, for 14 weeks throughout both observation periods. Our description of parturition in R. amplexicaudatus is based on data obtained from 16 females that were observed during the two sampling periods (Table 1). It was primarily constructed using partially observed parturition events. This is due, in part, to the fact that we filmed from outside the cave’s perimeter fences (in order to cause minimal disturbance to the bats), which forced us to rely on long-distance scanning of the colony to locate females of interest. However, the considerable size and density of the colony also made locating pregnant females difficult. Females did not segregate themselves during the parturition process. Instead, they gave birth amongst large groups of bats that were engaged in ordinary daytime activities, such as grooming and mating. Occurring within all of this behavioral noise, which was maintained at consistently high levels throughout the day, parturition turned out to be an extremely cryptic event (Image.1). Additionally, there were no large-scale visual cues associated with parturition that would indicate that labor was imminent. Consequently, in most cases, we were able to identify females in labor only by seeing the pups after they had already partially emerged. In seven observations we recorded data from emergence of the pup’s first body part to post-emergence (n=7, duration = 42:19, 40:46, 43:10, 81:42, 75:17, 40:54, 08:49 mm:ss). There were two observations where we recorded data from emergence of the pup’s first body part to the point where the female moved out of view (n=2, duration = 10:18, 69:47 mm:ss). There were four observations made after the emergence of the placenta (n=4, duration = 39:25, 25:40, 02:15, 30:21 mm:ss). However, despite these challenges, we did observe the parturition processes of three females prior to the emergence of their pup’s first body part (n=3, duration = 43:52, 43:51, 159:48 mm:ss).
Pre-parturition
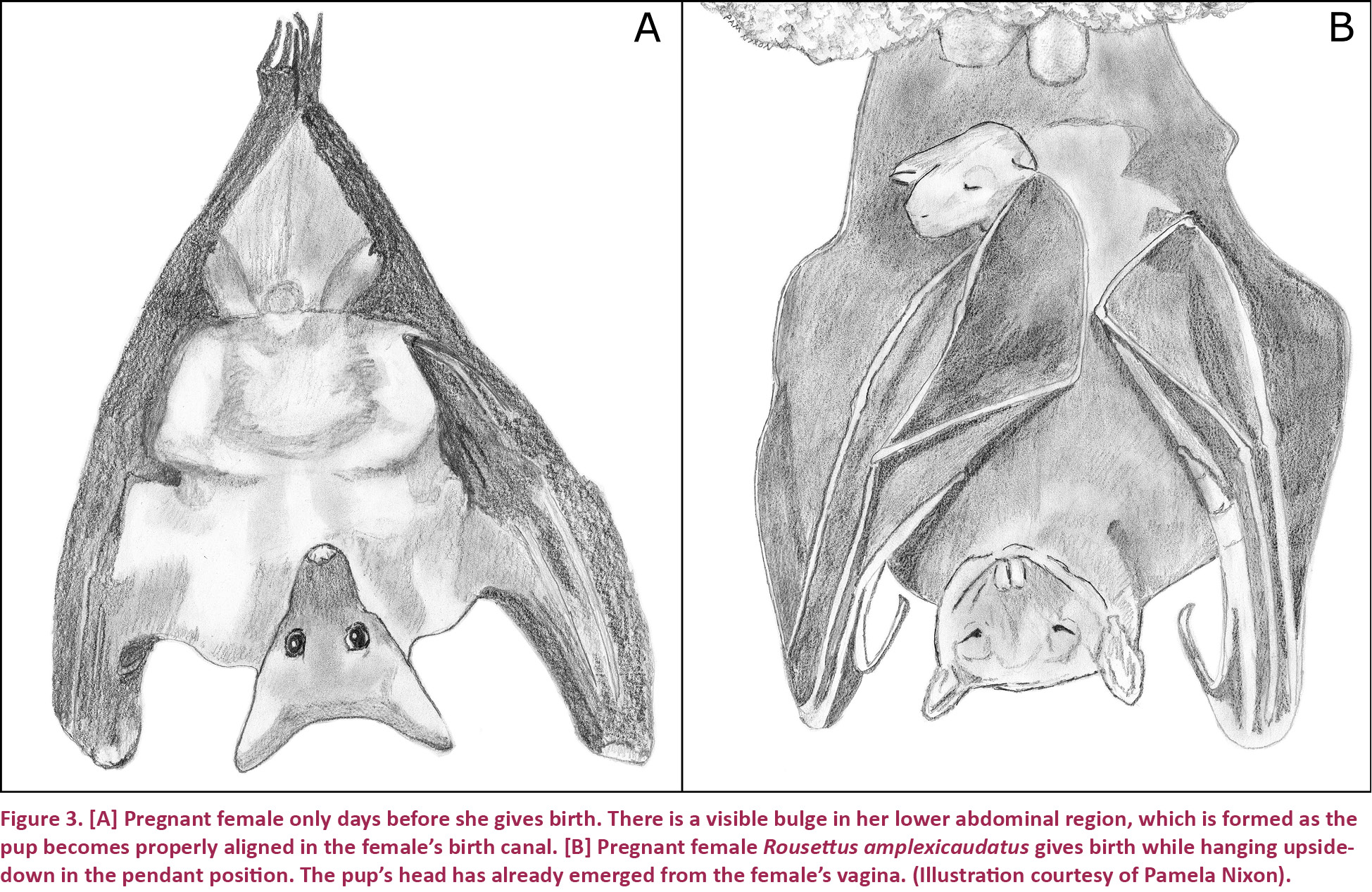
During the last few days of a female’s pregnancy, the pup became properly oriented within its mother’s uterus; the fetus settled into a position that facilitated its normal birthing pattern. This produced a visible bulge in the female’s lower abdominal region (Fig. 3A), making it very easy to visually identify females that were in the latest stage of their pregnancies. During this time, females appeared to seek out specific locations in the cave within which they would give birth. For example, the majority of pregnant females sampled gave birth at the second entrance of the cave, while no birthing events were observed at the cave’s fifth entrance. Additionally, the females appeared to actively maintain positions among the other bats that allowed their lower abdominal regions to remain unobstructed during labor. This served to prevent plant matter, rocks, and/or neighboring bats from blocking the pup during its delivery.
Parturition
Some bat species give birth in a heads-up position (Myotis lucifugus, Wimsatt 1945; Lasionycteris noctivagans, Kurta & Stewart 1990), while others hang from their thumbs (Pteropus rodricensis, Kunz et al. 1994). Additionally, some species, including R. amplexicaudatus, give birth while hanging upside down in a pendant position (Fig. 3B). All 16 pregnant females that we observed in this study gave birth in this position. Interestingly, very few mammals are adapted to giving birth against the forces of gravity; this is a specialization limited primarily to bats and sloths (McCrane 1966; Goffart 1971).
During labor, females alternated between having contractions and resting. During contractions, the females’ abdomens “hardened” and we could visually see the tightening and relaxing of the uterine muscles; this allowed us to identify discrete contractions. Interestingly, we observed two different contraction patterns. Some females had series of contractions in which the series were separated by large resting periods, usually several minutes in length, while the contractions themselves were rapid and typically spaced only seconds apart. For example, in one observation, a pregnant female displayed a series consisting of 14 contractions separated by the following intervals: 8, 5, 5, 4, 9, 4, 6, 12, 15, 9, 9, 6, and 13 seconds. There was a 2 minute 28 second period of rest separating this series of contractions from the preceding series and a one minute 58 second gap between it and the subsequent series. Other females, however, exhibited singular contractions that were each separated by large spans of time, typically around two minutes. For instance, one pregnant female experienced six independent contractions, each separated by the following time intervals (mm:ss): 2:10, 1:53, 1:57, 1:50, and 1:54.
In the majority of the birthing events observed (92%), pups were born in the head first presentation (Table 1; Video 1). When born in this position, the ventral side of the pup’s body always faced toward the ventral side of the female’s body. The pup was not encased in the amniotic sac upon emerging from the vagina, indicating that the sac ruptured earlier in the birthing process. In the head first presentation, the most difficult part of the birthing process, for the female, involved pushing the pup’s shoulders and wings out of the vagina, as they are the widest part of any bat’s body. It was not uncommon for a pup’s wrist(s) to become trapped inside the birth canal. This was a situation that extended the length of a birth, but did not seem to cause harm to the mother or pup. In one instance, a pup’s right wing had emerged from the vagina and become fully extended, while the entirety of the left wing was still inside the birth canal.
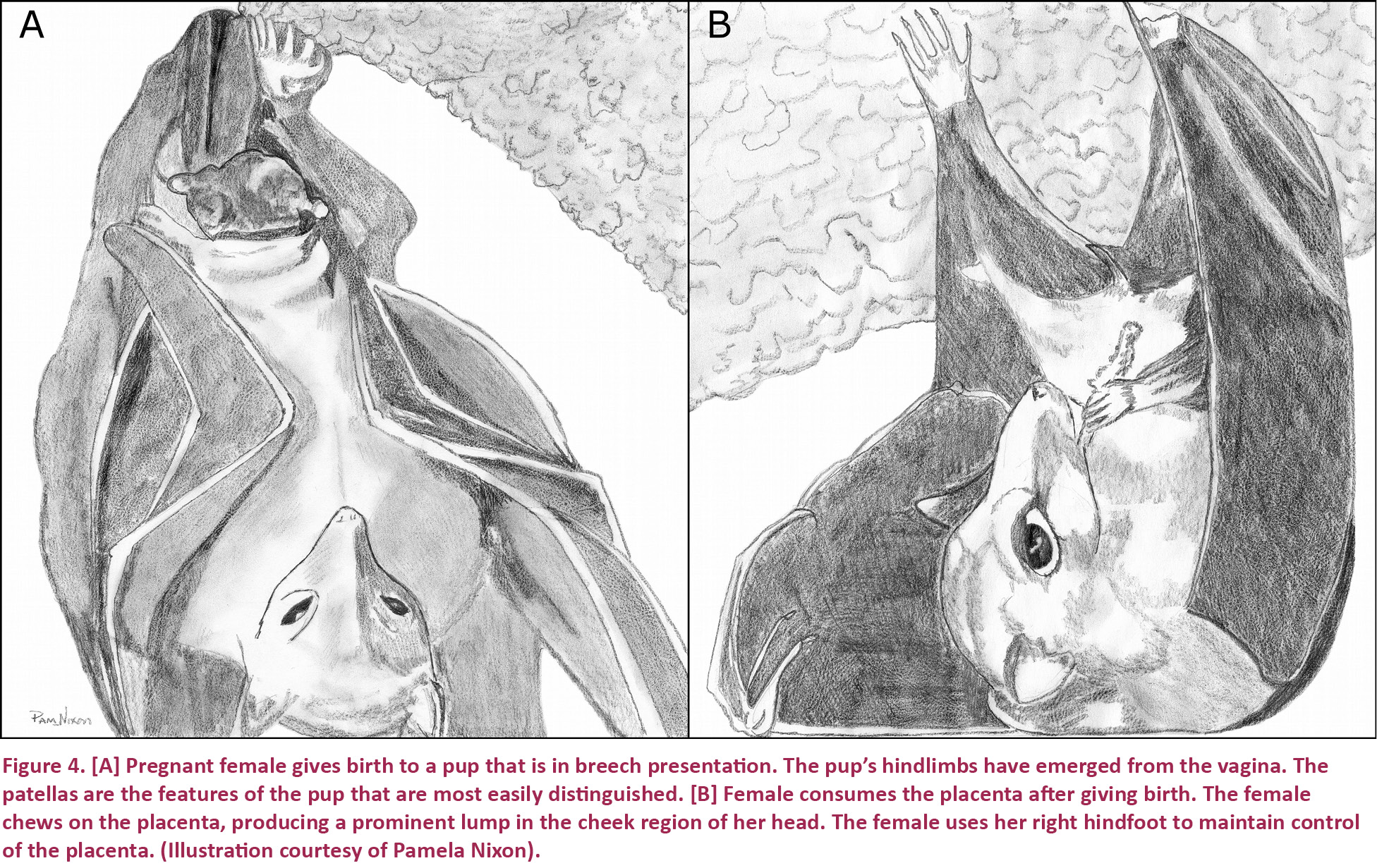
Only once was a pup was born tail first, in the breech presentation (Fig. 4A). This does not appear to be the normal presentation for this species. When breech presentation occurred, the pup’s elbows became trapped inside the birth canal, causing the female to expend greater amounts of time and energy in delivering the pup. In this situation the female exhibited greater distress. She was more active, shuffling from foot to foot and using her thumbs to assist in repeatedly changing her posture, and she spent more time licking both her vagina and the pup throughout the birthing process. During contractions the female assumed an open-mouth position as she strained to push out the pup. In contrast, the pup displayed reduced amounts of activity. Unlike those born in the head-first presentation, this pup became active only during the final minutes of the birth when it began moving its hindlimbs and feet. Before the female’s final contraction, the pup’s body was suspended from the female, hanging solely from its head, which had yet to emerge from the vagina. It is unknown if the pup’s survival was effected by having been born in the breech presentation. The female and pup remained visible for 31 minutes and 15 seconds following the end of the birth. At this point in time, the pup was still alive and active.

Post-parturition
Once both of the pup’s wings emerged from the vagina, the birthing process finished quickly. The time that passed between this point and the end of the birth was oftentimes only seconds in length, the average being 14 seconds (n=9). As the pup fully emerged, the female used her wings to assist in catching and orienting it. Afterwards, the female enclosed the pup within her wings, presumably for the purposes of thermoregulation, bonding, and protection from nearby bats. The female immediately began cleaning the pup and herself with her tongue. Meanwhile, the pup searched for one of the female’s nipples. As the female cleaned, she continuously jostled the pup, which made locating a nipple challenging. In one instance, a female was so energetic in her attempts to clean the pup that it was pushed from one nipple to the other five times within the span of 25 seconds. The female’s cleaning activities were interrupted by resting periods of varying lengths. As a part of these cleaning activities, the female ate the pup’s nutrient-rich placenta (Fig. 4B). We did not observe attempts by any female to immediately sever a pup’s umbilical cord after giving birth. Instead, the cord remained attached to the pup for a long period of time following the birth, usually greater than 30 minutes. The average that we observed was 34 minutes and 8 seconds (n=11). The umbilical cord simply ceased to perform any function after the female consumed the placenta, detaching the cord from any other structures. It is easy to spot females that have recently given birth, due to the fact that the skin around their vaginas retains a stretchy and distended appearance in the first few days following the event.
Unlike many mammals, female R. amplexicaudatus neither isolated themselves during birth nor did they receive aid from others. Females gave birth amid the rest of the bats that make up the colony, an estimated 1.8 million individuals. A female in labor was often surrounded by bats of all age classes, sexes, and reproductive stages. These other bats went about their normal routine, largely ignoring the female as she gave birth. Bats surrounding the female were observed grooming, sleeping, and even fighting. Sometimes a bat from another area of the cave landed near the female striking her as it did so. On occasion, this disturbance was so severe that the female was forced to move to a new location, even if it meant that she must fly to a different spot in the cave to do so. During this study, the cameras recorded video of a female forced to fly to a new location in the cave after her pup’s head had already emerged from her vagina (Video 2).
Description of the neonate
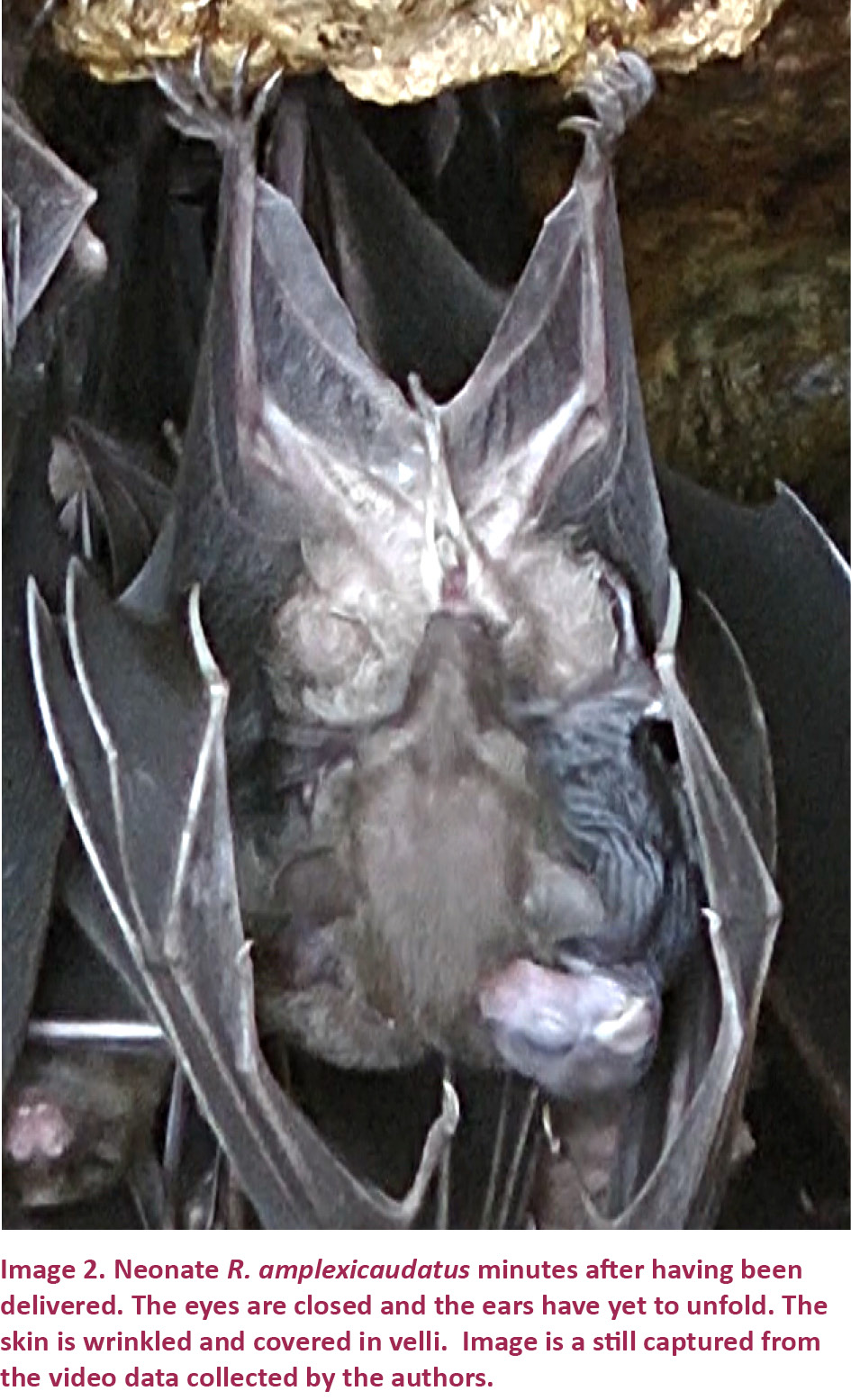
Neonate R. amplexicaudatus (Image 2) that we observed were entirely covered in light brown velli, through which the underlying pink skin was clearly visible. Young were born with their eyes closed. At birth, the ears were folded, but, within minutes of emerging from the vagina, they unfolded. Pups born in the head-first presentation were extremely active both during and after birth. After a pup’s head emerged from the vagina, the pup began yawning, licking, moving, and vocalizing. This high level of activity was sustained even after parturition was concluded. Following birth, the pup secured its hold on the female by placing one foot on either side of the vagina, near the juncture where each hindlimb meets the female’s torso, and used its wings to move about on the female’s chest, normally in search of her nipples. A result of their well-developed clinging abilities, neonate R. amplexicaudatus were capable of remaining with the female in flight. We observed many females flying with pups on their chests during our daytime observations and we also photographed a female flying with her pup during one of our observations of the colony’s daily outflight activities.
DISCUSSION AND CONCLUSIONS
This study has produced a descriptive model for the parturition process of R. amplexicaudatus, based on observations compiled from sixteen pregnant females. One of the most interesting findings is that female R. amplexicaudatus do not immediately sever their pups’ umbilical cords after birth, as happens in many species of mammals. This is not an uncommon phenomenon among bats and has been observed in a variety of species. Neonate Lasiurus cinereus, Euderma maculatum, Erophylla planifrons, and Tadarida cynocephali were all observed to have had intact umbilical cords 60 minutes after having been born (Sherman 1937; Jones 1946; Bogan 1972; Easterla 1976). Additionally, biologists observed a four-hour-old neonate Artibeus planirostris that was still attached to its umbilical cord (Jones 1946). It has been proposed that this is an adaptive strategy to giving birth while suspended and hanging upside-down (Bogan 1972). In the first few hours of its life, the pup is extremely vulnerable to losing its hold on the female. This is due to a combination of factors including the pup’s naturally high activity levels, jostling by the female, and disturbance by nearby bats and even predators. In the event that a pup does get displaced, an unsevered umbilical cord acts as a safety mechanism, preventing the pup from falling to its death. In 1937, Sherman observed a neonate Tadarida cynocephali lose its grip and dangle harmlessly from its un-severed cord (Sherman 1937). A similar situation was observed several times during this observation of R. amplexicaudatus.
As with most studies involving the observation of wild animals, there were certain limitations and challenges associated with obtaining the video data for this study. These primarily related to maintaining sight of pregnant females of interest. The animals were neither isolated nor housed in artificial environments where extraneous environmental variables could be controlled, so bats under observation frequently moved out of our fields of view. Visibility was obscured or completely eliminated when pregnant females became covered by other bats, moved behind rock formations or tree roots within the cave, and when they were disturbed by predators (ranging from feral cats and dogs to crows and snakes). For example, one pregnant female was visible for only 35 seconds. She was covered by a neighboring male both before and after that brief window. The pup’s head had already emerged from the vagina by the time the female became visible to us. However, during our window of visibility, we were able to record the emergence of the rest of the pup’s body and initiation of the female’s cleaning activities. This was a brief visual observation that was not recorded in our video data, as such it was not used to develop our parturition model. However, it serves to demonstrate how and why partial observations played such a significant role in the model’s creation.
An additional limitation was the penchant of female R. amplexicaudatus to cover newborn pups with their wings. This limited our ability to assess the activities of neonates in extraordinary detail. We also experienced a minor equipment limitation in this study. Our video cameras had excellent high definition capabilities, but they were not waterproof. Unfortunately, the research site was located in a tropical environment that experienced daily rain showers. As a result, we were sometimes forced to terminate data collection activities in order to ensure the long-term integrity of the camera equipment. Despite these challenges, we were able to develop a descriptive model of parturition, for a wild colony of bats in their natural environment, based on observations made on one of the largest samples of pregnant females of bats ever seen in scientific literature.
There exists enormous potential for additional studies to be carried out on this topic in the future. Given the amount of success seen at this particular cave, we believe that it would be worthwhile to perform a second study on parturition at the same research site. With more time and a larger number of cameras, it is conceivable that data could be obtained for a much larger sample of pregnant females. Furthermore, this second study could be designed to answer questions about seasonal variation in the reproductive patterns and output of this population of R. amplexicaudatus by comparing data temporally. It would also be interesting to conduct night time observations of the colony in order to assess whether or not parturition takes place at night and to determine if pups accompany the females during their nightly foraging activities or if they are left behind inside the cave.
Rousettus amplexicaudatus is a common species with an extensive geographic range (Fig. 1). It is neither globally threatened nor endemic (Csorba et al. 2008). Generally, this leads us to believe that the species is stable and of minimal conservation concern. However, R. amplexicaudatus is negatively impacted by deforestation, cave disturbance, climate change, introduction of invasive predators, and overharvesting by humans throughout its range (Mickleburgh et al. 2002; Wiles & Brooke 2009). Deforestation, which is particularly severe in the Philippines (Jones et al. 2009), limits vegetative cover, results in the patchy distribution of food resources, and has the potential to destroy important navigational landmarks (Mickleburgh et al. 2002). Cave disturbances, resulting from activities such as guano mining and visitation by tourists, render otherwise suitable caves unsuitable for habitation, which limits an already finite resource (Mickleburgh et al. 2002; Jones et al. 2009). Meanwhile, climate change has the potential to alter existing vegetative communities (Hughes et al. 2012) and generate more severe weather systems, causing increased bat mortality (Wiles & Brooke 2009). Finally, predation by invasive predators (such as feral cats and dogs) and humans (who harvest bats for bushmeat, sport, medicine, and trade) place R. amplexicaudatus under even greater levels of strain (Mickleburgh et al. 2002; Wiles & Brooke 2009). In some locations, the disturbances caused by these various threats have been so severe that the species has completely abandoned many of its historic roosts (Carpenter et al. 2014). Biological residues (e.g. staining on the cave walls) and anecdotal information are the only evidence that the roosts were once inhabited by this species (Carpenter et al. 2014).
So, despite its broad distribution and the perception that it is quite common, R. amplexicaudatus faces profound systemic, ecological stress throughout its range. We are concerned that the presence of multiple large roosts masks, or even causes biologists to overlook, critical signs of stress, such as systematic roost abandonment, in the species. It might be the case that these few conspicuous roosts overshadow important trends seen in the greater number of smaller roosts and deceive biologists into thinking that the species is healthier than it really is. Essentially, we run the risk that these “showy” roosts dominate our attention and serve to convince us that the species is thriving. It is not an exaggeration to hypothesize that the trajectory of this species may follow that of other “common” species that were widespread, gregarious, and ignored until ecological stressors caused their rapid and irreversible collapse (e.g., Ectopistes migratorius, the passenger pigeon, and Conuropsis carolinensis, the Carolina parakeet). As a result, early research, such as that provided in this study, may prove invaluable in the stabilization and long term conservation of this species. We hope that this study will provide baseline data for this species, which can be used to formulate hypotheses that can be tested in the field, at different roost sites, in order to determine if all populations of R. amplexicaudatus adhere to these findings.
Since females are the only sex physically capable of producing offspring it is important that as much as possible is known about their specific reproductive requirements, especially when it comes to conservation. This study helps biologists gain a better, more thorough, understanding of how female R. amplexicaudatus give birth and the space allocation requirements necessary for them to perform this activity. For example, in many bat species, pregnant females segregate themselves during parturition, even to the point of needing distinct maternity roosts (Pteropus poliocephalus, Nelson 1965; Nycticeius humeralis, Watkins & Shump 1981; Miniopterus minor, McWilliam 1990). The fact that pregnant female R. amplexicaudatus observed in this study did not segregate themselves is of particular note. If this pattern is maintained throughout the species range, it could have powerful management implications in that it might mean that fewer roosts are necessary to maintain viable populations of this species.
The more we know about the natural history of R. amplexicaudatus, the more effectively populations can be monitored, managed, and if the need arises, conserved. As we have demonstrated, the reproductive biology of a species plays an integral role in its status and can have important conservation implications. Effective bat conservation plans are those that take into account the unique reproductive strategies of the species they are attempting to conserve, as every species has distinctive reproductive rates, disturbance susceptibilities, and roost requirements. We know that there are immense gaps in our knowledge of the biology, ecology, and population statuses of many bat species. Fortunately, conducting this study allowed us to identify some of the largest gaps in our knowledge of the reproductive biology of R. amplexicaudatus. Tropical bats like R. amplexicaudatus have lives that are very seasonally-driven. Consequently, environmental cues play important roles in the timing of particular events and behavior. We need to determine the environmental mechanisms that play a role in the reproductive behavior of this species and the ways in which they trigger or halt certain aspects of reproduction. Additionally, we should seek to identify the relationship between population size and reproductive success in R. amplexicaudatus. By better understanding optimal population size for this species, we can monitor populations to ensure that mortality rates do not exceed recruitment rates. Biologists know that R. amplexicaudatus tend to roost in large numbers and that the largest colony can be found at Monfort Bat Cave, but we know neither the optimal colony size that the species is adapted for nor do we have record of historical population sizes. Consequently, it is difficult for us to accurately determine the stability of populations of R. amplexicaudatus. The findings of this study are meant to serve as a foundation for future exploration. Building on our results by carrying out addition ecological studies in the future, will allow us to begin answering many of the questions we have about the biology of this species.
REFERENCES
Baudinette, R.V., S.K. Churchill, K.A. Christian, J.E. Nelson & P.J. Hudson. (2000). Energy, water balance, and the roost microenvironment in three Australian Cave-dwelling Bats (Microchiroptera). Journal of Comparative Physiology B 170(5–6): 439–446; http://dx.doi.org/ 10.1007/s003600000121
Bergmans, W. (1997). Taxonomy and biogeography of African Fruit Bats (Mammalia: Megachiroptera). Beaufortia 47(2): 11–90.
Blake, H.A. (1885). Note on the parturition of a West-Indian bat. The Scientific Proceedings of the Royal Dublin Society 4(9): 449–450.
Bogan, M.A. (1972). Observations on parturition and development in the Hoary Bat, Lasiurus cinereus. Journal of Mammalogy 53(3): 611-614.
Bradbury, J.W. (1977). Lek mating behavior in the Hammer-headed Bat. Ethology 45(3): 225–255; http://dx.doi.org/10.1111/j.1439-0310.1977.tb02120.x
Carpenter, E., R. Gomez, D.L. Waldien & R.E. Sherwin (2014). Photographic estimation of roosting density of Geoffroy’s Rousette Fruit Bat Rousettus amplexicaudatus (Chiroptera: Pteropodidae) at Monfort Bat Cave, Philippines. Journal of Threatened Taxa 6(6): 5838–5844; http://dx.doi.org/10.11609/JoTT.o3522.5838-44
Csorba, G., G. Rosell-Ambal & N. Ingle (2008). Rousettus amplexicaudatus. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. <www.iucnredlist.org>. Downloaded on 02 October 2012.
Derrickson, E.M. (1992). Comparative reproductive strategies of altricial and precocial eutherian mammals. Functional Ecology 6(1): 57–65.; http://dx.doi.org/10.2307/2389771
Downs, N.C., V. Beaton, J. Guest, J. Polanski, S.L. Robinson & P.A. Racey (2003). The effects of illuminating the roost entrance on the emergence behaviour of Pipistrellus pygmaeus. Biological Conservation 111(2): 247–252; http://dx.doi.org/10.1016/S0006-3207(02)00298-7
Easterla, D.A. (1976). Notes on the second and third newborn of the Spotted Bat, Euderma maculatum, and comments on the species in Texas. American Midland Naturalist 96(2): 499–501; http://dx.doi.org/10.2307/2424094
Giannini N., A. Goswami & M.R. Sánchez-Villagra (2006). Development of integumentary structures in Rousettus amplexicaudatus (Mammalia: Chiroptera: Pteropodidae) during late-embryonic and fetal stages. Journal of Mammalogy 87(5): 993–1001; http://dx.doi.org/10.1644/06-MAMM-A-016R1.1
Goffart, M. (1971). Reproduction and development, pp. 142–160. In: Alexander, P. & Z.M. Bacq (eds.). Form and Function in the Sloth. Pergamon Press, Elmsford, New York, USA, 225pp.
Heideman, P.D. & R.C.B. Utzurrum (2003). Seasonality and synchrony of reproduction in three species of nectarivorous Philippines bats. BMC Ecology 3: e11; http://dx.doi.org/10.1186/1472-6785-3-11
Hood, W.R., J. Bloss & T.H. Kunz (2002). Intrinsic and extrinsic sources of variation in size at birth and rates of postnatal growth in the Big Brown Bat Eptesicus fuscus (Chiroptera: Vespertilionidae). Journal of the Zoological Society of London 258(3): 355–363; http://dx.doi.org/10.1017/S0952836902001504
Hoying, K.M. & T.H. Kunz (1998). Variation in size at birth and post-natal growth in the insectivorous bat Pipistrellus subflavus (Chiroptera: Vespertilionidae). Journal of the Zoological Society of London 245(1): 15–27; http://dx.doi.org/10.1111/j.1469-7998.1998.tb00067.x
Hughes, A.C., C. Satasook, P.J. Bates, S. Bumrungsri & G. Jones (2012). The projected effects of climatic and vegetation changes on the distribution and diversity of Southeast Asian bats. Global Change Biology 18(6): 1854–1865; http://dx.doi.org/10.1111/j.1365-2486.2012.02641.x
Jones, K.E., S.P. Mickleburgh, W. Sechrest & A.L. Walsh (2009). Global overview of the conservation of island bats: importance, challenges, and opportunities, pp. 496–530. In: Fleming, T.H. & P.A. Racey (eds.). Island Bats: Evolution, Ecology, and Conservation, 1st Edition. The University of Chicago Press, Chicago, Illinois, USA, 549pp.
Jones, T.S. (1946). Parturition in a West-Indian Fruit Bat (Phyllostomidae). Journal of Mammalogy 27(4): 327–330; http://dx.doi.org/10.2307/1375339
Keeley, A.T.H. & B.W. Keeley (2004). The mating system of Tadarida brasiliensis (Chiroptera: Molossidae) in a large highway bridge colony. Journal of Mammalogy 85(1): 113–119; http://dx.doi.org/10.1644/BME-004
Kunz, T.H., P.V. August & C.D. Burnett (1983). Harem social organization in cave roosting Artibeus jamaicensis (Chiroptera: Phyllostomidae). Biotropica 15(2): 133–138; http://dx.doi.org/10.2307/2387958
Kunz, T.H., A.L. Allgaier, J. Seyjagat & R. Caligiuri (1994). Allomaternal care: helper-assisted birth in the Rodrigues Fruit Bat, Pteropus rodricensis (Chiroptera: Pteropodidae). Proceedings of the Zoological Society of London 232(4): 691–700; http://dx.doi.org/10.1111/j.1469-7998.1994.tb04622.x
Kunz, T.H. & S.K. Robson (1995). Postnatal growth and development in the Mexican Free-tailed Bat (Tadarida brasiliensis mexicana): birth size, growth rates, and age estimation. Journal of Mammalogy 76: 769–783; http://dx.doi.org/10.2307/1382746
Kurta, A. & T.H. Kunz (1987). Size of bats at birth and maternal investment during pregnancy. Symposia of the Zoological Society of London 57: 79–106.
Kurta, A. & M.E. Stewart (1990). Parturition in the Silver-haired Bat, Lasionycteris noctivagans, with a description of the neonates. The Canadian Field-Naturalist 104(4): 598–600.
Manalo III, V. & T. Cinco (2011). Climate of the Philippines. https://web.archive.org/web/20120224124110/http://kidlat.pagasa.dost.gov.ph/cab/statfram.htm. Accessed on 13 January 2012.
McCrane, M.P. (1966). Birth, behaviour, and development of a hand-reared Two-toed Sloth, Choloepus didactylus. International Zoo Yearbook 6(1): 153–163; http://dx.doi.org/10.1111/j.1748-1090.1966.tb01733.x
McCracken, G.F. & G.S. Wilkinson (2000). Bat mating systems, pp. 321–362. In: Crichton, E.G. & P.H. Krutzsch (eds.). Reproductive Biology of Bats. Academic Press, London, England, 510pp.
McWilliam, A. N. (1990). Mating system of the bat Miniopterus minor (Chiroptera: Vespertilionidae) in Kenya, East Africa: a lek? Ethology 85(4): 302–312; http://dx.doi.org/10.1111/j.1439-0310.1990.tb00409.x
Mickleburgh, S.P., A.M. Hutson & P.A. Racey (2002). A review of the global conservation status of bats. Oryx 36(1): 18–34; http://dx.doi.org/10.1017/S0030605302000054
Nelson, J.E. (1965). Behaviour of Australian Pteropodidae (Megachiroptera). Animal Behaviour 13(4): 544–557; http://dx.doi.org/10.1016/0003-3472(65)90118-1
Ness, S.A. (2003). Where Asia Smiles: An Ethnography of Philippine Tourism. University of Pennsylvannia Press, Philadelphia, Pennsylvannia, USA, 328pp.
Ramakrishna, P.A. (1950). Parturition in certain Indian bats. American Society of Mammalogists 31(3): 274–278; http://dx.doi.org/10.2307/1375293
Rossiter, S.J., G. Jones, R.D. Ransome & E.M. Barratt (2000). Parentage, reproductive success and breeding behaviour in the Greater Horseshoe Bat (Rhinolophus ferrumequinum). Proceedings of the Royal Society of London 267(1443): 545–551; http://dx.doi.org/10.1098/rspb.2000.1035
Sherman, H.B. (1937). Breeding habits of the Free-tailed Bat. Journal of Mammalogy 18(2): 176–187; http://dx.doi.org/10.2307/1374464
Simmons, N.B. (2005). Order Chiroptera, pp. 312–529. In: Wilson, D.E. & D.M. Reeder (eds.). Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd Edition. Johns Hopkins University Press, Baltimore, Maryland, USA, 2142pp.
Springer, M.S., E.C. Teeling, O. Madsen, M.J. Stanhope & W.W. de Jong (2001). Integrated fossil and molecular data reconstruct bat echolocation. Proceedings of the National Academy of Sciences 98(11): 6241–6246; http://dx.doi.org/10.1073/pnas.111551998
Stone, E.L., G. Jones & S. Harris (2009). Street lighting disturbs commuting bats. Current Biology 19(13): 1123–1127; http://dx.doi.org/10.1016/j.cub.2009.05.058
Thomas, D.W., M.B. Fenton & R.M.R. Barclay (1979). Social behavior of the Little Brown Bat, Myotis lucifugus. Behavioral Ecology and Sociobiology 6(2): 129–136; http://dx.doi.org/10.1007/BF00292559
Twente, Jr. J.W. (1955). Some aspects of habitat selection and other behavior of cavern-dwelling bats. Ecology 36(4): 706–732; http://dx.doi.org/10.2307/1931308
Vaughan, T.A. & R.P. Vaughan (1987). Parental behavior in the African Yellow-winged Bat (Lavia frons). Journal of Mammalogy 68(2): 217–223; http://dx.doi.org/10.2307/1381460
Watkins, L.C. & K.A. Shump, Jr. (1981). Behavior of the evening bat Nycticeius humeralis at a nursery roost. American Midland Naturalist 105(2): 258-268; http://dx.doi.org/10.2307/2424744
Webb, P.I., J.R. Speakman & P.A. Racey (1992). Oxygen consumption and evaporation during parturition in a vespertilionid bat (Pipistrellus pipistrellus). Journal of Reproduction and Fertility 94(2): 525–528; http://dx.doi.org/10.1530/jrf.0.0940525
Wiles, G.J. & A.P. Brooke (2009). Conservation threats to bats in the tropical Pacific islands and insular Southeast Asia, pp. 405–459. In: Fleming, T.H. and P.A. Racey (eds.). Island Bats: Evolution, Ecology, and Conservation, 1st Edition. The University of Chicago Press, Chicago, Illinois, USA, 549pp.
Wimsatt, W.A. (1945). Notes on breeding behavior, pregnancy, and parturition in some vespertilionid bats of the eastern United States. Journal of Mammalogy 26(1): 23–33; http://dx.doi.org/10.2307/1375029